In this interview, News Medical talks to Dr. Thomas Mueller of Bruker Nano surface, about the advances in BioAFM.
BioScope Resolve is your newest BioAFM. Its name suggests a focus on spatial resolution. What can this new AFM resolve that other AFMs cannot?
BioScope Resolve has enabled the first AFM images resolving individual microvilli on live cells. This is remarkable in that atomic force microscopy has been widely used to image live cells, and microvilli are often present. Yet the surface of those cells had remained featureless in AFM images.
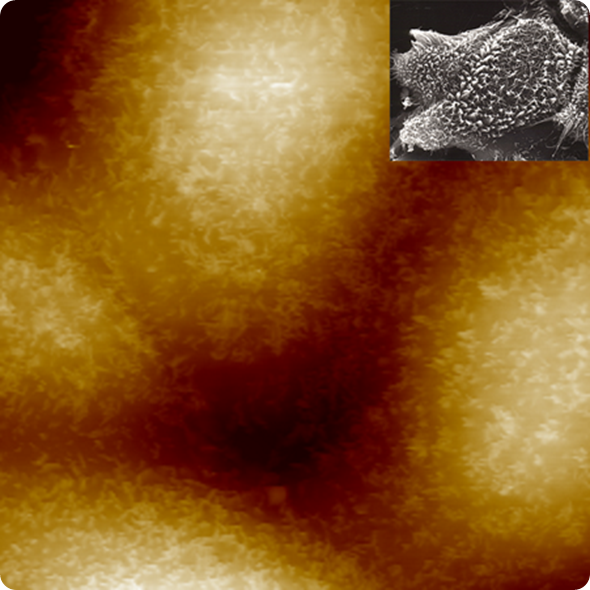
1kHz PeakForce Tapping image of live MDCK cells, Dr. Hermann Schillers, University of Münster. (Inset: SEM image of MDBK cells at 2400x mag.)
We now understand that PeakForce Tapping® plays a key role in achieving this breakthrough. With PeakForce Tapping on the BioScope Resolve system, imaging is controlled directly by piconewton force at a precise location, avoiding both, lateral and spatially averaged forces. This is different from TappingMode, where forces throughout the tapping cycle contribute to feedback.
A discussion about spatial resolution would be incomplete without touching on biomolecular imaging. Given how much high-resolution AFM imaging of individual biomolecules has been explored already, what would BioScope Resolve allow that hasn’t been done already?
With BioScope Resolve, we can routinely resolve the double helix structure of DNA, including both major and minor groove, while on the inverted optical microscope.

The Hoogenboom group has shown that with AFM, and in particular with a Bruker AFM with PeakForce Tapping one can resolve the double helix structure so well at the individual molecule level that one can see deviations from the average structure.
With BioScope Resolve one can now envision new structure-function studies: Tracking the distortion of local DNA structure in action, for example, during protein binding – while concurrently using fluorescence to identify and track the chemical moieties involved.
The Biophysics research community has witnessed an increasing interest in biomechanics at the single cell level, for example, as a diagnostic tool in cancer detection. Where do you see the strongest contribution of atomic force microscopy in this field and what does Resolve bring to it?
Atomic force microscopy may be the key tool to achieve a deeper, more complete, and more quantitative understanding of the mechanical response of cells to their environment, as a function of disease state.
It is becoming clear that an AFM can provide very accurate, quantitative data, when the whole range of system and probes issues are addressed.
Probes need to be calibrated by vibrometer to guarantee the required accuracy. Tips need to be long enough to avoid squeeze film effects. The mechanics of biosample carriers must be considered in avoiding large noise contributions. System dynamics must be fast while maintaining sufficient Z-range to probe whole cells.
This is in a nutshell what we did when developing BioScope Resolve and its tailored set of probes.
Going beyond the elastic response, how does BioScope Resolve address the time- or frequency-dependent mechanical response of a cell, its viscoelasticity?
BioScope Resolve offers the widest range of tools for probing viscoelasticity and with it the time- and rate-dependent mechanical response of live cells.
As force curves are at the center of AFM mechanical probing, it starts with the widest range of ramp rates available. With our FASTForce Volume and PeakForce Tapping technologies, we span sub-hertz to kilohertz ramp rates, while maintaining piconewton force control.
As shown in the ability to image microvilli, we really do maintain piconewton force control even at the fastest kilohertz rates.
Complementing all this, we have recently introduced a very flexible ramp scripting capability to BioScope Resolve. This allows direct cell relaxation measurements, taking advantage of the fast dynamics and accurate force control to quantify relaxation times.
The scripting function includes the ability to sweep a modulation frequency. This opens the door to dynamic mechanical analysis on live cells, probing the mechanical response spectrum. Ultimately this is the information one needs to determine the storage and loss modulus at the relevant frequency range.
On BioScope Resolve, all this functionality is integrated into force volume, allowing whole data cubes of relaxation times and dynamic response to be created.
What do you think the future holds for BioAFM?
Cell mechanobiology and receptor mapping on live cells are certainly growing areas where atomic force microscopy can play an even larger role. Especially where, with the right control algorithms, it can play out its unique advantage of probing forces quantitatively at nanometer scales.
This is why we started our BioScope Resolve development with a focus on highest resolution and sensitivity, and built an extensive set of force mapping tools on this foundation.
For atomic force microscopy to play out this strength it needs to integrate with fluorescence. This has driven our development of extensive optical synchronization and navigation capabilities and will continue to inform our development of new AFM capabilities.
Where can readers find more information?
We have been working hard to make our website, www.bruker.com/AFM, a resource for atomic force microscopy, rather than just a marketing location. The site has webinars, application notes, galleries, and descriptions of modes and techniques. And of course it has detailed information on BioScope Resolve and our other AFMs.
About Dr. Thomas Mueller
.jpg)
Dr. Thomas Mueller is the Director of Product Management in the AFM business unit of Bruker’s Nano Surfaces Division. Thomas has been with Bruker for 12 years, having held positions in applications and product management, and is the author of over 50 publications, reviews, and application notes.
He received his Ph.D. in 2000 from Yale University on developing new linear and nonlinear spectroscopic probes of molecular structure and dynamics, followed by postdoctoral research at Columbia University focusing on scanning probe microscopy as a tool for interrogating self-assembly and chemical reaction specificity.