May 16 2016
K2M Group Holdings, Inc., a global medical device company focused on designing, developing and commercializing innovative and proprietary complex spine and minimally invasive spine technologies and techniques, today announced that research on K2M’s RAVINE® Lateral Access System will be presented at the SpineWeek 2016 Annual Meeting, occurring May 16–20 in Singapore.
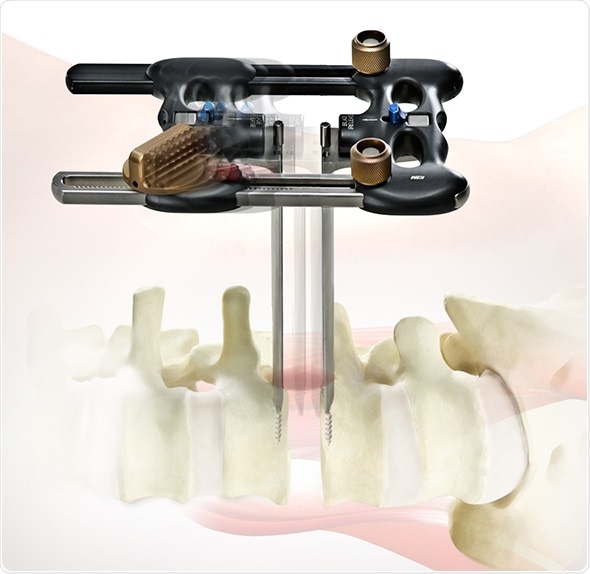
“The RAVINE Lateral Access System allows for a lateral approach to lumbar fusion, and the dual flat blade platform provides a secure means for accessing the disc space with increased visualization compared to traditional tubular lateral retractors,” said Mr. Robert Lee, presenting author of four RAVINE-focused studies and Consultant Spine Surgeon at the Royal National Orthopaedic Hospital in Stanmore, United Kingdom. “The four studies featured at SpineWeek help establish RAVINE’s potential value to surgeons treating degenerative spinal deformities through a true muscle-splitting transpsoas approach. RAVINE provides surgeons with minimally invasive options to correct anterior column malalignment whilst providing indirect neural decompression across multiple levels.”
Data from four RAVINE studies will be presented at SpineWeek 2016:
- Clinical and Radiological Results following Lateral (LLIF) Versus Transforaminal Lumbar Interbody Fusion (MI-TLIF), Lee R., Sedra F., Wilson L., Afsharpad A., Dala-Ali B: (Oral Presentation #283, Wednesday 18 May, 17.08, Room 5)
- Early Outcomes in the Use of Minimally Invasive Lateral Cages in Primary Adult Degenerative Scoliosis Correction Surgery – Minimum 6 Month to 2 Year Follow-Up, R.S. Lee: (Oral Presentation #361, Thursday 19 May, 16.34, Room 5)
- Accuracy of Pre-operative Surgical Planning in Predicting Postoperative Alignment in Patients Undergoing Minimally Invasive Multilevel Anterior Column Reconstruction for Positive Sagittal Balance; R.S. Lee: (Oral Presentation #460, Friday 20 May, 10.24, Room 4)
- Early Outcomes in the Use of Minimally Invasive Lateral Cages in Lumbar Revision Surgery, Minimum 6 Month to 2 Year Follow-Up; R.S. Lee: (SMISS Poster Presentation #20)
“K2M is pleased with the breadth of RAVINE research being shared at SpineWeek 2016,” said Eric Major, President and CEO of K2M. “We believe these data complement our focus on innovation within the global spinal market as evidenced by RAVINE’s compatibility with the CASCADIA™ Lateral Interbody System featuring Lamellar 3D Titanium Technology™, K2M’s innovative and proprietary technology that uses 3D printing with the goal of allowing for bony integration throughout an implant.”
The RAVINE Lateral Access System is a dual flat blade platform for a true muscle-splitting transpsoas approach that offers rigid fixation to the spine and an option for both a third and fourth blade. K2M’s lateral access system represents an innovative design departure from the tubular retractors, while providing tremendous adaptability to both patient anatomy and surgeon technique.
For more information on the RAVINE Lateral Access System, as well as K2M's complete product portfolio, visit www.K2M.com.
About K2M
K2M Group Holdings, Inc. is a global medical device company focused on designing, developing and commercializing innovative complex spine and minimally invasive spine technologies and techniques used by spine surgeons to treat some of the most difficult and challenging spinal pathologies. K2M has leveraged these core competencies to bring to market an increasing number of products for patients suffering from degenerative spinal conditions. These technologies and techniques, in combination with a robust product pipeline, enable the Company to favorably compete in the global spinal surgery market.
Source: www.K2M.com