PENTAX Medical launches a world first for endoscopy, the OPTIVISTA EPK-i7010 Video Processor, featuring both digital and optical enhancements, in the European, Middle Eastern & African (EMEA) markets. This unique enhancement combination provides detailed information for more accurate endoscopic in vivo diagnosis through improved vessel and mucosal pattern characterization.
The new OPTIVISTA EPK-i7010 video processor uniquely features both digital and optical enhancements for more accurate endoscopic in vivo diagnosis through improved vessel and mucosal pattern characterization. (Images courtesy of Dr. Federico Buffoli, Ospedale di Cremona, Italy)
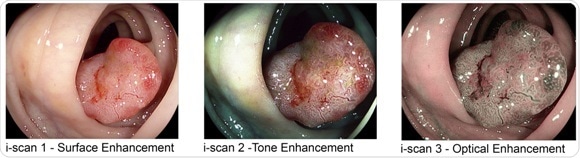
The introduction of OPTIVISTA offers physicians both digital (i-scan Surface and Tone Enhancements) and Optical Enhancement (i-scan OE), with the new optical filter producing bandwidth-limiting light. When combined with image enhancement processing technology i-scan OE clearly displays the surface structures of blood vessels, glandular ducts and mucosal membrane in higher contrasts than white light. This unique combination with the proven digital i-scan technology, in one video processor, provides an enhanced view of the mucosal structures and vascular patterns, supporting early detection, demarcation and characterization. Physicians can switch seamlessly in real time amongst HD+ white light and three i-scan modes which includes i-scan OE to view multiple aspects of tissue structure.
PENTAX Medical’s new OPTIVISTA EPK-i7010 video processor, together with its latest i10 HD+ endoscopes series, or with its MagniView, optical zoom endoscopes, provides market leading image quality. Perfectly suited to routine procedures or complex interventions, the OPTIVISTA is designed to support the optimal clinical outcome.
Dr Silvia Sanduleanu, Maastricht UMC+, The Netherlands, who has been involved in early studies with OPTIVISTA said:
Contrast enhancement using i-scan OE highlights the epithelial mucosal surface pattern and especially the vascular pattern in detail. i-scan OE facilitates detection and in vivo diagnosis of lower gastrointestinal tract pathology enabling targeted endotherapy.
In addition to improved diagnosis, OPTIVISTA is a powerful teaching platform. TwinMode is useful in teaching the appropriate interpretation of image enhanced endoscopy, providing simultaneous comparison of side by side endoscopic images. The simultaneous comparison of enhanced clinical images is particularly useful in demonstrating the appropriate characterization of lesions.
Also included with the OPTIVISTA is the ability to perform video recording, enabling the capture of HD+ video files onto a USB storage device. Audio recording for video is captured through an external microphone. The new, intuitive touch screen controls allow for simple and efficient operation. For optimum image collection, the OPTIVISTA incorporates freeze scan technology which automatically selects the sharpest picture for users’ records. By integrating the video and audio capture functionality there is no requirement for further recording devices or software.
Rainer Burkard, President EMEA of PENTAX Medical, commented:
This is just one of a series of exciting product launches for PENTAX Medical this year. We are looking to increase our support to consultants across GI, ENT and bronchoscopy by adding to our product portfolio over the coming months. This is a proud moment for us as a business, OPTIVISTA will have a marked effect on the diagnosis possibilities for consultants. Working with our other technologies we are in a strong position to support the entire clinical pathway, from initial identification through to therapy.