Macrophages are frontline cells in our immune system. They detect microbial invaders and also tissue injury and then mount an appropriate response needed to clear the infection and repair the damaged tissue.
We have found that during macrophage activation with the bacterial product LPS, the mitochondria undergo profound metabolic changes. In particular, we have found that they stop making ATP (their regular role) and start to make reactive oxygen species.
So instead of using oxygen to generate ATP, they use it to generate reactive oxygen species, which in turn leads to activation of a transcription factor called HIF1alpha. This drives expression of inflammatory genes such as that for IL-1beta, a central driver of inflammation.
We have found the underlying mechanism of this complex process – a metabolite called succinate is oxidised by the enzyme SDH, and this in turn leads to reactive oxygen species generation by the mitochondria in a process called reverse electron transport.
We have found that by inhibiting SDH you can prevent the macrophage from making inflammatory factors, and instead they make anti-inflammatory ones, so we think this could be useful as a means of turning down macrophages when they are over-active, as happens in inflammatory diseases and sepsis.
Which inflammatory diseases is the elevated response thought to be implicated in?
Sepsis, but also we think many inflammatory diseases, such as rheumatoid arthritis, osteoarthritis, inflammatory bowel disease and diseases of the CNS such as MS. This is because macrophages are implicated in all of these conditions.
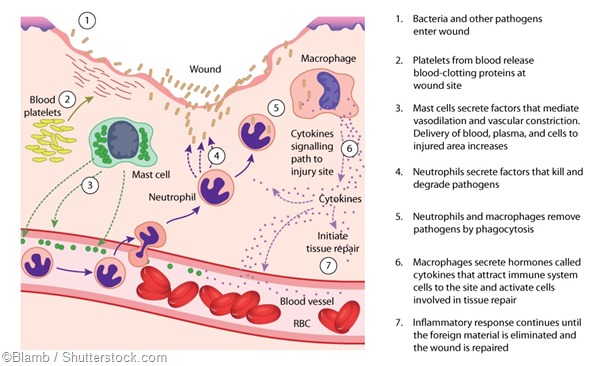
Do you think it will be possible to find ways of suppressing macrophages to reduce associated tissue damage when the body’s inflammation is over the top?
Yes – we think that by blocking SDH the macrophages will start behaving themselves, and will be beneficial, limiting the damage that occurs in inflammatory diseases.
What impact do you think your research will have on the field of immunology and the area of immunometabolism?
Our paper joins a growing body of literature which is all pointing in the one direction – that profound and complex metabolic changes are occurring in inflammatory cells and that it might be possible to target these changes for therapeutic gain.
How long is it likely to be before patients can ultimately benefit from this increased understanding of inflammation via new therapeutic options?
Ah…the big question! It will take some time, although there are already drugs in use that target metabolism such as metformin and methotrexate so these lead the way. It is likely that new drugs will be developed, but this will take years to reach patients. That sadly is the way of things!
What do you think the future holds for immunometabolism?
More and more fascinating discoveries will emerge that will provide more insights into the inner working of our immune systems in health and disease.
Immunometabolism has effectively become another important aspect to immunity, along with other areas such as cytokines and pattern recognition receptors.
Where can readers find more information?
A recent review I co-wrote with Jeff Rathmell and Rigel Kishton – O’Neill LA, Kishton, R and Rathmell J (2016) Nature Reviews Immunology 16, 553-565.
About Professor Luke O’Neill, PhD FRS
Professor Luke O’Neill holds the Chair of Biochemistry at Trinity College Dublin, where he leads the Inflammation Research Group.
He has a PhD in Pharmacology from the University of London and carried out Post-Doctoral research at Cambridge U.K. on the pro-inflammatory cytokine IL-1 and innate immune signalling.
His research is in the area of the molecular basis to inflammatory diseases, with a particular interest in Toll-like receptors, inflammasomes and metabolic control of inflammatory cell signalling.
He has won numerous awards for his research, notably the Royal Irish Academy Medal for Biochemistry, the Royal Dublin Society/ Irish Times Boyle medal for Scientific Excellence and in 2014 the European Federation of Immunology Societies Medal.
He was elected a member of EMBO in 2005. In 2016 he was named by Thompson Reuters as one of the world’s most influential scientists, being in the top 1% in Immunology.
He is a co-founder of 2 Spin-out companies from his lab - Opsona Therapeutics, a drug development company working in the area of Toll-like receptors, and Inflazome, which is developing inhibitors of inflammasomes for inflammatory diseases.
He was elected a Fellow of the Royal Society in 2016.