An interview with Mike Clayton, Abbott, conducted by April Cashin-Garbutt, MA (Cantab)
How important are diagnostic tests in clinical decision making?
Diagnostic tests are critical as up to 60 to 70% of critical clinical decisions are influenced by diagnostic test results. It's of huge importance to the running of any healthcare system.
Here at Abbott, we believe that providing clinicians with quick access to diagnostics information and insights can help them make quick clinical decisions around the appropriate treatment for a patient, which is important for good patient outcomes. For me, it all starts with a rapid and accurate diagnostic test.
_-_680x_.jpg)
Image Credit: Abbott
What was Abbott’s vision behind Alinity?
Alinity was just over five years in the making. We spent a long time talking to both laboratory professionals and physicians because we wanted to observe them at work to understand their challenges and listen to their feedback so we could design an overall solution – not just an instrument – that helps them provide high quality diagnostics in the most efficient way.
We understood that healthcare providers were facing a large number of unprecedented challenges, such as needing higher testing volumes and increased flexibility. Issues such as staffing, lack of space, and complex, time intensive processes and instruments present ongoing challenges.
Having spent a lot of time understanding those aspects, we recognised that we needed to develop a series of solutions for both instrumentation and the IT and service support side (which helps provide insights), to ensure that we could address our customers’ challenges.
The result of these efforts is Alinity, our new unified suite of systems, which has effectively been designed and shaped to meet the needs of our lab and healthcare professionals. . In particular, Alinity is designed to help improve operational productivity in the lab. The goals are faster delivery of test results, more automated processes that make it easier for staff to manage, and helping busy laboratories do more with fewer resources.
_-_680x_-1.jpg)
Image Credit: Abbott
What additional challenges had to be overcome?
After understanding the challenges our healthcare providers and laboratory professionals faced, we knew that one we had to overcome was the design of diagnostic testing systems across related specialities, such as clinical chemistry, immunoassay, point-of-care, hematology and molecular diagnostics. We asked how we could achieve a common user interface, that would then be easy to use.
Within that, one particular challenge was the development of instruments that could produce more test results without requiring a larger footprint; one of the biggest dilemmas laboratory professionals described was the need to do more, but in a space that was only going to become smaller.
Finally, the other main challenge we had to overcome was delivering these benefits without lots more hands-on training being required for laboratory professionals.
Those are three good examples of challenges that we had to overcome in the design and development, whilst also having to properly understand what challenges our customers were facing in order to deliver better patient care.
How did you arrive at the name Alinity?
The name was born out of three key attributes we offer, which are 'alignment', 'innovation' and 'unity'.
By merging these three words, we are referring to how the suite of products is ‘aligned’ to the clinicians' and laboratory professionals' goals; aimed at providing ‘innovative’ solutions to address the challenges they meet every day, and finally, how the harmonization of the systems and the IT suite, provides ‘unity’ in allowing those challenges to be met.
These three key elements or attributes have been woven into the name. This ties in nicely with our vision of what we're trying to achieve, which is helping these laboratories and hospital institutions improve the performance of the lab and contribute to better outcomes for patients.
Can you please give an overview of the different Alinity products?
For the first time, lab professionals and physicians will have a unified, family of systems across laboratory disciplines. The Alinity family includes point of care, immunochemistry, haematology, molecular and blood screening systems, and features common operational functionality and increasing confidence and efficiency of diagnostic testing.
An advantage of the Alinity family of systems is what we call a “lock and key” design of the different reagent bottles; it’s impossible to put the wrong bottle in the wrong slot in the door of the instrument. If you think about the benefits of minimising human error and therefore being far more efficient with staffing and making it easier to train laboratory professionals in using the system effectively, what that does is help deliver fast, efficient and accurate results of the highest quality. You can therefore get results to the clinician faster, while doing so within a smaller footprint.
The CE mark for Alinity ci, which is the clinical chemistry and immunoassay system, was announced in January 2017. It is designed to help labs run more tests in less time, reduce human error, increase test productivity and help a laboratory be far more efficient in quickly delivering chemistry or immunoassay results to the clinician, so they are able to make a better and quicker decision.
One of the key challenges discussed was that, traditionally, and with diagnostics at the moment, the two major aspects of the instruments – specimen sample processing and the loading and storage of reagents – are side-by-side. Firstly, this means the instrument is physically quite large, and, secondly, if you want to reload reagents, you have to pause the instrument, lift up the lid, and replace them.
With the Alinity ci system, we have overcome this by providing a stacked carousel design, where one sits above the other, rather than side-by-side. The sample processing is on the top and the reagent loading and storage are on the bottom.
There are three key benefits to this design. Firstly, it means you can do more testing within a smaller footprint and it provides a scalable design to allow flexibility for the changing needs of the laboratory as volumes shift.
Secondly, when you open the door of the Alinity ci system, there's a reservoir you can load reagents into while the instrument is running, so you don't have to pause it. This improves speed and productivity.
The increased loading capacity for samples and tests with the ci system means that if a stack of samples come in, but one is an emergency sample from A&E that needs the result turned around very quickly, instead of having to use two designated fast lanes, you can use any of the lanes on the front of the system itself. Simply pushing a button tells the instrument that a particular sample in a lane is a STAT emergency sample, so again, you're not interrupting the everyday laboratory workflow and you are getting results back to the clinicians quickly when it is key.
Finally, the design of the Alinity ci is very intuitive and the interface is user-friendly. This is common across all of the Alinity platforms. If you imagine that within the one lab you have one team of professionals operating the chemistry or immunoassay systems and then a different team operating the hematology instrumentation, with a common user interface, you can have the same team easily trained in operating both systems.
This is because the system is designed to be intuitive from a user interface perspective, and since some of the key features such as the lock and key design of the reagent bottles are common to all the instruments, you can be far more streamlined in how you use staff and therefore improve that operational productivity side.
In January of this year, we also announced CE mark for Alinity s, which is for blood and plasma screening. As with any Alinity platform, it has the same user interface and method of use. Some other key features this system has are the capacity to run up to 600 tests per hour in a compact footprint and the user has the ability to walk away from the instrument for atleast three hours after loading samples, while they are being analyzed.
In blood and plasma screening, laboratory professionals find having the opportunity to move away and do other things, without having to keep returning to the instrument, a key benefit. You can have continuous access to samples and supplies and reload without pausing and stopping the instrument.
Another member of the Alinity family is the i-STAT Alinity, for which we received CE mark at the end of November 2016. The i-STAT Alinity is a hand-held, portable blood testing system, that allows a large menu of blood tests from a single device, from different types of blood chemistries to different types of cardiac markers, but all using just two or three drops of blood at the patient’s side.
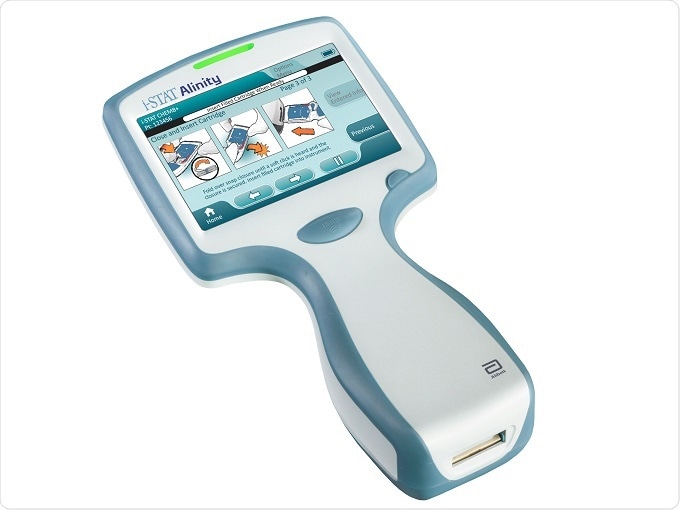
Image Credit: Abbott
You can get a test result into the clinician's hands in anything between two and ten minutes. If you are looking after a patient that might not necessarily be in a hospital, for example, but you want a quick result on a cardiac marker, you can get a rapid result there and then and make the right and informed treatment decision quickly.
With the i-STAT Alinity, you can be wireless or it can be hard-wired, so healthcare providers can manage the data from a central point and hub. There's also the potential for things like cloud connectivity, which uses standard browsers and makes it very easy for the health systems to customize the i-STAT Alinity instrument. This provides a really nice solution, where you have a hand-held, portable i-STAT Alinity device that can sit hand-in-hand with the larger core laboratory and be flexible about addressing those different diagnostic testing needs, whether it be within a core laboratory or out at an offshoot doctor's surgery, for example.
At the same time, all would have the same user interface, making it very easy for the operator to use. Again, this offers clinicians better information more quickly, enabling them to make quicker, better decisions to improve patient outcomes.
One of the key elements in terms of improving operational productivity and helping laboratories improve patient outcomes is AlinIQ. Alinity is the instrumentation, and AlinIQ, which we launched last year, is a suite of professional services and software tools to help labs adapt to their most pressing challenges. If you have very high quality data being quickly generated by the instruments, AlinIQ helps convert that information into intelligent insights. It's those insights that enable you to bring huge value to the laboratory, both within the laboratory and in terms of the impact that the lab can have on its whole healthcare system.
AlinIQ is a suite of professional services and informatics that can be broken down into four areas. The first is its proactive and preventative services, which we call the AlinIQ Always On Services. This offering provides predictive alerts to labs and enables the detection and prevention of potential instrument downtime, up to three days in advance. By keeping an eye on and monitoring specific information, AlinIQ can help predict if an instrument may go down within three days, which allows us to do some very quick preventative work to ensure the instrument doesn't go down. You're therefore keeping that continuity of service. If an instrument should go down, it can be a lengthy process to get it back up again.
Next there are data harmonization services, called the AlinIQ Analyser Management System or AMS. This tool provides labs and hospital networks with the ability to integrate different software systems into a single standardized platform. That offering helps optimize sample processing time and workflow efficiency across the instrument, while at the same time producing quality results.
Then there are the operational services called the AlinIQ Business Intelligence System or BIS. This offering helps optimize the capacity of existing systems to help the busy labs absorb different volume increases, without the need for additional budget. This tool, which is very much hand-in-hand with our experts, helps provide business intelligence, expertise and outcomes. For example, it's a great tool to better align the right staffing across the full working day and at the right times, depending on the sample volumes. It's one very simple way of ensuring you're neither overstaffed, nor understaffed no matter what point in the day.
The final arm to AlinIQ is inventory management and optimisation, which is called the AlinIQ Inventory Management System or IMS. That tool combines innovative RFID technology with streamlined processing consulting, which allows labs to get ahead on things like operational needs. It helps laboratories ensure that they have the right volume of correct reagents at the appropriate time within their organisation. As well as being crucial in helping minimise shortages, this tool has a huge impact in helping reduce waste and also takes out the time involved in potentially lengthy manual processes.
If you think of AlinIQ being used in a dovetailed way with Alinity, along with our experts and professional services, this is particularly crucial in helping those laboratories maximise and achieve the better patient outcomes and performance that they're looking to do.
Do health institutions need all the different systems or can they work in isolation and in conjunction with other systems?
They can work in isolation, but you start to get the true benefits when you link them together and also build in some of the AlinIQ aspects to deliver those insights.
If you can deliver one test more quickly, for example, laboratories then say "Well, we want all our testing delivered more quickly,” not just one area, but in chemistry or in immunoassay, for example.
Are all the products now available?
At the moment, we've made CE mark announcements for i-STAT Alinity, Alinity s, and the Alinity ci series.
Alinity h, which is the hematology instrument, and Alinity m, which is for molecular diagnostics, are expected to announce CE marks later this year.
What feedback have you received from users of the Alinity instruments so far?
The early feedback has been met with excitement and I think customers have been surprised by how small the footprint is, given the volumes that are coming out of it.
How do Abbott plan to update the Alinity systems moving forward?
That's a good question. Abbott will continue to build the testing menu and will add more and more tests throughout the year. For me, one of the key aspects here is that this is just the start of a journey in terms of rolling out and making this system with Alinity and AlinIQ a reality for our customers.
One of the things that has worked really well was taking the time to truly understand the challenges that were being faced by laboratories and hospital institutions. In terms of other, further developments, we're ensuring that we stay really close to the laboratory professionals so that we can continue to make sure that, firstly, Alinity meets the challenges they're facing now and, secondly, meets the evolving challenges that may not be fully understood yet.
What do you think the future holds for diagnostics?
Diagnostics has a really bright future as it continues to become increasingly important within hospital institutions in helping them deliver better patient outcomes and better healthcare performance.
We're excited about continuing to work very closely with labs and helping to make them more efficient and able to have a greater impact on patient outcomes.
Where can readers find more information?
Alinity
References
- WHO Global Health Expenditure Atlas, September 2014. World Health Organization: https://www.who.int/.
- Global Health and Aging Report. National Institute on Aging and the World Health Organization: https://www.nia.nih.gov/research/publication/global-health-and-aging/preface.
- Forsman, RW. Why is the laboratory an afterthought for managed care organizations? Clinical Chemistry. 1996; 45(5): 813-16.
About Mike Clayton
 Mike joined Abbott’s Diagnostics Division as Managing Director of the Region North (United Kingdom, Ireland, Denmark, Norway, Sweden and Finland) in April 2016.
Mike joined Abbott’s Diagnostics Division as Managing Director of the Region North (United Kingdom, Ireland, Denmark, Norway, Sweden and Finland) in April 2016.
Over his 21 years of service at Abbott, Mike has held a number of senior posts, most recently as General Manager for UK and Ireland for Abbott’s Diabetes Care Division. He has also headed the Rheumatology, Marketing, Sales, and Business Development teams within the company.
His responsibilities have included leading, motivating and developing a staff of more than 150 employees, alongside strategically planning for both brand and corporate milestones and overseeing the delivery of financial reviews.
Milestone successes of Mike’s career to date include the successful launch of Freestyle Libre in the UK & Ireland, and leadership of the team that led the Prime Minister and Home Secretary to visit the company. Alongside these achievements, Mike also built and executed a major Government Affairs program within the company.
Mike also led the UK Direct-to-Consumer Freestyle InsuLinx campaign, which won him the company’s Divisional Vice President’s Award in December 2012.
Mike received his bachelor’s degree in Biomedical Sciences from the University of Sunderland.