Sep 12 2017
Researchers at Okayama University describe in Scientific Reports that cancer-associated fibroblasts — cells that play a key role in cancer progression — originate from cancer stem cells. Preventing cancer stem cells from transforming into cancer-associated fibroblast may be a promising approach towards cancer treatment.
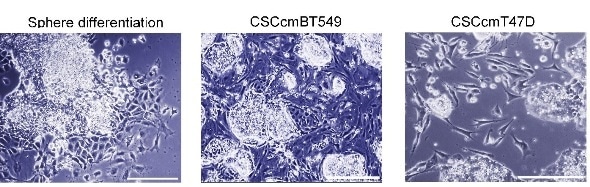
Representative bright field images showing the morphology of cells differentiating from cancer stem cell spheres.
Cancer progression is partly governed by a specialized type of cells known as cancer-associated fibroblasts (CAFs), as they have the ability to support/mediate different signaling pathways in a tumor microenvironment. However, it is still unclear that how CAFs are generated. Now, a team of researchers led by Masaharu Seno from Okayama University has provided the first evidence that CAFs may originate from cancer stem cells (CSCs) — the cells that can develop into any type of cell occurring in a given tumor. An important implication of this finding is that novel therapeutic strategies can be designed to inhibit CSC-to-CAF conversion limiting the cancer progression
Seno and colleagues first created CSC-like cells following a protocol they had established earlier: by exposing the mouse induced pluripotent stem cells (miPSCs) , a type of reprogrammed cells with embryonic–like pluripotent state which can differentiate into any type of cells, to the conditioned medium prepared from the culture of human breast cancer cell line. The resulting cells displayed typical CSC-like phenotype. These cells exhibited three essential features of CSCs. The first one is self-renewal, which is attributed to a potential to form spheres in serum-free suspension cultures. The second one is ability to form malignant tumors in vivo. And the last one is the potential of differentiation. Here in this case the phenotype of CAF is found as one of the phenotypes CSCs differentiate into. The researchers then separated fibroblast-like cells differentiating from CSC-like spheres in the presence of conditioned medium. These cells were compared with fibroblast-like cells generated directly from miPS cells. Comparative analysis revealed that CSC generated fibroblast-like cells displayed CAF-like phenotype. Therefore, Seno and co-workers concluded that the conditioned medium plays a key role in the differentiation of CSC-like spheres into CAFs.
Finally, the expression of CAF markers (proteins that are associated with the formation of CAFs) were analyzed and scientists found that the CAFs have high invasive potential when compared with normal fibroblasts. Therefore, these findings by Seno and colleagues indicate that CSCs are a source of CAF-like cells in tumor microenvironment. Their model system is a valuable tool for analyzing the role of CAFs derived from CSC-like cells in the tumor microenvironment and, in the words of the researchers, “inhibiting the conversion of CSCs to CAFs might have potential therapeutic implications in the future”.
Source: http://www.okayama-u.ac.jp/eng/