Oct 31 2017
Researchers at Okayama University describe in the Journal of Neuroscience that a certain protein known to play a major role in circadian rhythmicity — humans’ intrinsic 24-hour biological cycle — is also key to proper brain functioning. The findings may increase our understanding of neurological diseases and guide the development of future treatments.
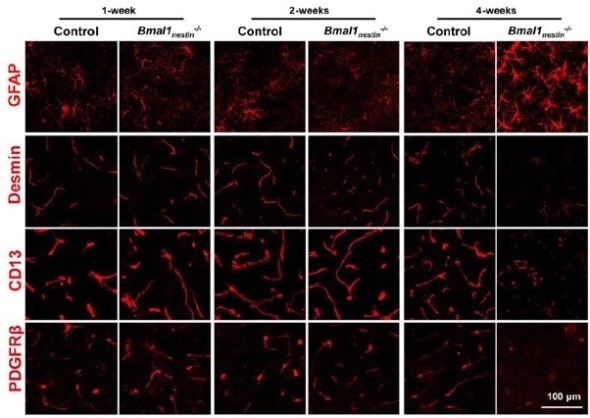
Confocal microscopy images showing the evolution of astrocyte activity (GFAP; increasing with age) and pericyte marker expression (Desmin, CD13, PDGFRβ; decreasing with age) in mice with Bmal1 deficit.
Patients suffering from psychiatric or neurological diseases often have irregular sleep patterns. In fact, neurodegenerative diseases, such as Alzheimer’s, are believed to be caused by a disturbed biological response to the day/night cycle (a.k.a. circadian rhythm). A team of researchers led by Takeshi Takarada from Okayama University has now identified a link between circadian rhythm disturbance and brain dysfunction. The scientists showed that a protein called Bmal1, known to play an important role in circadian rhythmicity, also regulates the stability of the blood–brain barrier (BBB), a semipermeable membrane in the brain that separates blood from other, extracellular fluid.
Takarada and colleagues made their discovery by studying the function of Bmal1 in transgenic green-fluorescent-protein (GFP) mice. The introduction of GFP is a commonly used biomedical technique for obtaining fluorescence microscopy images — in this case, of the brains of mice. First, the researchers found that deletion of Bmal1 molecules results in an increased activity of astrocytes, a type of cell in the brain that provide biochemical support to the BBB. They then observed that Bmal1 deficiency leads to higher-than-normal permeability of the BBB due to pericyte dysfunction. Pericytes are cells needed to sustain proper BBB function; they regulate capillary blood flow.
As to the origin of the pericyte dysfunction causing reduced integrity of the BBB, the scientists were able to show that Bmal1 deletion affects the expression of platelet-derived growth factor receptor β (PDGFRβ) proteins in pericytes, which leads to a decrease in pericyte coverage of blood vessels in the brain. This reduced coverage was found to be age-dependent.
The work of Takarada and colleagues clearly establishes a connection between circadian rhythms and the physiological stability of the BBB: Bmal1 is involved in both. The scientists, therefore, concluded that:
… Bmal1 may represent a novel target for the discovery and development of therapies for many neurodegenerative and/or psychiatric disorders related to abnormal BBB integrity.
Source: http://www.okayama-u.ac.jp/