
An interview with Prof. Hermann Schillers, Universität Münster conducted by April Cashin-Garbutt, MA (Cantab).
Can you please give a brief introduction to your research?
I run a core facility for AFM techniques, biological medical applications. My research is focussed on in the interaction of platelets and cancer cells.
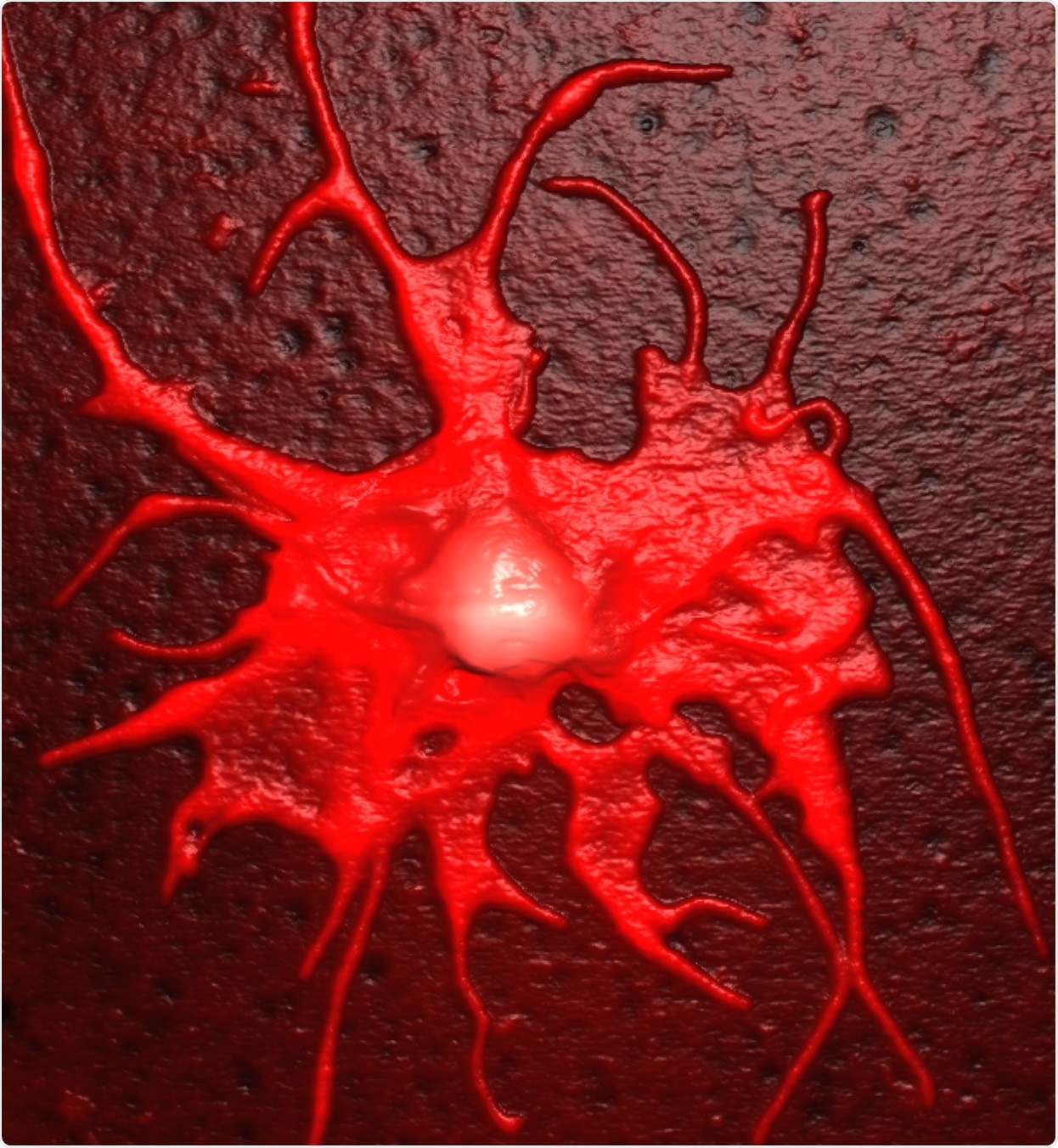
Platelets support cancer cells in nearly every step of forming metastases, starting with the escape of immune surveillance, followed by the cancer cell arresting to the vessel wall and also in extravasation.
Our idea is that if we could prevent the interaction of platelets and circulating cancer cells, we may find something to block the formation of metastases, which might fight cancer.
How do you use AFM imaging and force-spectroscopy-based modes to study the structure and mechanical properties of cancer cells?
In platelet−cancer cell interaction, I use single cell force spectroscopy to quantify the interaction of platelets and cancer cells and to quantify the effect of drugs that prevent this interaction.
With this technique, we got real numbers of the forces between platelets and cancer cells for the first time. Microfluidic experiments allow to quantify the number of platelets on cancer cells, but we can't quantify the strength of that interaction. With single cell force spectroscopy we got picoNewtons (pN) and femtoJoules (fJ). And this is necessary when you want to know which drug prevents this interaction at which concentration.
Another point is that we want to see what happens when a platelet binds to a cancer cell. The actual understanding of the situation is that platelets form a kind of ´invisible´ cloak around the circulating cancer cell, but we never observed this.
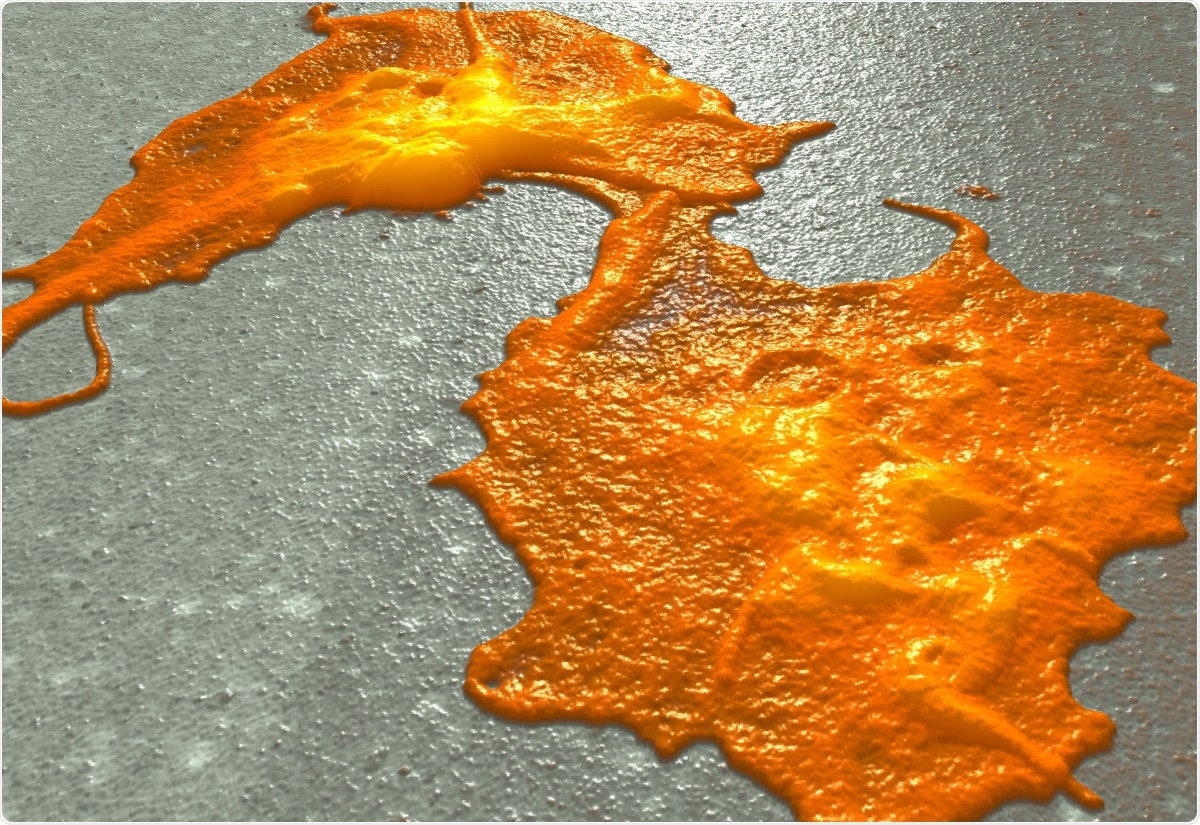
When we scan platelet cancer cell aggregates, we see that platelets on top of cancer cells vanish within 30 minutes. Using Resolve's fastTapping mode, we could observe this uptake of platelets into cancer cells. We proved this with fluorescent techniques such as cell sorting and confocal microscopy and we clearly saw that there was an uptake but not a cloak formation.
How much is currently known about the way in which cancer cells “hijack” the services of platelets?
The widespreaded view is the cloak formation of platelets around circulating cancer cells. This layer of platelets protects the cancer cell against the immune system and, in the next step, facilitate the arrest of the cancer cell-platelet aggregate to the endothelial wall to start the extravasation.
Since we never observed this cloak formation but always observe the uptake of platelets by cancer cells, we think it is more likely that the cancer cell uses platelet proteins, platelet-specific adhesion molecules, to adhere to the vessel wall and also to escape immune surveillance.
In addition, it is known that platelets, as well as platelet-derived microparticles, contain mRNA and this changes the proteome of the cancer cell. Therefore, it could be both ways: the use of platelet proteins directly after the platelet uptake, followed by use of the platelet mRNA to produce platelet proteins to escape immune surveillance and adhere to the vessel wall.
In what way can AFM further our understanding?
We look for the first step of platelet cancer cell interaction, but there are several further steps. What I now try to do is to use PeakForce QNM to see a pattern of biomechanical changes of the cancer cell after interaction with platelets.
Studying Tumor-Platelet Interactions using AFM
Studying Tumor-Platelet Interactions using AFM from AZoNetwork on Vimeo.
So far we found a kind of biomechanical footprints at the position where the cancer cell gobbles up a platelet. There is a change in elasticity and viscosity in the cancer cell membrane that may give us some hints about the mechanism by which the cancer cell incorporates the platelet. We know from experiments that this is in a dynamin-dependent process but want to know more about this.
The next step is that we want to know what change the cancer cell undergoes after uptake of platelets. We are again using single cell force spectroscopy. We incubate cancer cells with platelets and then perform single cell force spectroscopy on activated endothelium.
We are looking for the arrest of circulating cancer cells on endothelium and quantify the adhesion force. We saw that when cancer cells made contact with platelets, they became much stickier to the activated endothelium, compared to untreated cancer cells. That is one of the projects we are currently involved in.
We also look for the interaction site of these platelet-incubated cancer cells on activated endothelium. I don't think that the platelet-incubated cancer cell could adhere everywhere on activated endothelium. There must be a specific predilection site.
We want to figure out where it adheres to. Is this cell junction or the cell body of endothelial cells or does the endothelial cell need special mechanical characteristics to form a metastatic nice with a platelet-incubated cancer cells and then proceed to extravasation?

What is the biggest impact that AFM has made to the biological and nanomedicine research fields?
We are able to look at living cells and even at subcellular structures. It is still a technique where we are limited to the surface, but when we apply a little bit force, we can see beneath the surface and observe cytoskeletal dynamics, for instance.
Twenty years ago, there were images of a couple of cells, rather blurry than resolved, and then it started to become more and more a high resolution imaging technique for living cells. However, pure imaging of cells runs out of steam after a while mechanical characterization became the focus and there is still a focus on the measurement of live cell's elasticity, viscoelasticity and the changes in the elastic modulus. Cell dynamics is something we could follow with AFM and it includes biomechanics as well as biochemistry.And biomechanics is as important as biochemistry.
The field of biochemistry is 30 or 40 years old, with very detailed knowledge about the biochemistry of cells, but biomechanics is rather new. In 2007, Michael Sheets and Viola Vogel published a review where they showed that mechanics influenced biochemistry and vice versa. An example: When you apply force to a protein it might open up cryptic binding sides or destroy binding sites, then the intracellular signalling pathway and the cells behaviour goes in a different direction. Today we know that biomechanics influences biochemistry and biochemistry influences biomechanics.
How has Bruker technology helped or advanced AFM in biological research?
There's the tapping mode, which was invented by Bruker many years ago allowing to get information about the cell − curvature, cell heights, providing morphological data.
The latest great success was PeakForce QNM where we get several data sets of live cells` morphology and biomechanical parameters, such as elasticity, dissipation, adhesion and deformation in one scan process.
And this at a rather good speed, especially on the Resolve system, which enabled us to observe dynamical changes of a layer of cells, single cells and even subcellular structures like cytoskeletal rearangements with a time resolution in the range of a minute and below. So perhaps you may not have significant changes in the topographic channel, but you did see a lot in the data channels for adhesion, dissipation or elasticity. This is a real improvement.
What is the importance of meetings, like the AFM BioMed Conference, to you and the AFM research community?
One might say that today you could use Skype, e-mail or a phone to get in contact with the people, but that's not the same – it is completely different. When we met for the conference, we stayed here together for a couple of days.
A well-organized coffee break is the most important thing at a meeting because people come together to talk about things they don't come up with when they are on stage giving a talk. So the time outside the lecture hall is very important, people discussing so many things and longlasting contacts start here.
Many, many cooperations start from meetings like this. No Skype conference will ever substitute a conference where people physically come together to talk, so it is absolutely necessary.
What direction do you see, or would like to see, AFM going in the next five years? (What do you see as the next big thing for AFM?)
One of the things AFM needs is speed. More speed would mean that you could follow the dynamics of cells at a better time resolution. Another point is that since AFM was invented we have used the old optical lever system and I think it is now time for a new system that moves away from one cantilever, or one indenter, to a multi-probe array AFM.
Several ideas are being discussed and micro- and nanofabrication techniques have reached a high level of development. So why should the leading providers of AFMs not try to improve AFM techniques by a more fundamental change?
Such a multi-probe array would be interesting not only in terms of speed and resolution, but also in terms of measuring biomechanical characteristics. The way we do it today is we indent the cell in this position, that position, and so on.
But we know that a cell reacts on each indentation which causes calcium spikes and cytoskeletal rearrangement; what we get in the last indentation is different from what we get in the first indentation. Therefore, multi-probe array system would be very useful for every AFM user and especially in the field of Bio-AFM application.
Another thing that was mentioned during the talks at AFM BioMed was chemical characterization. We are still in a situation where we are blind-folded when we touch our samples. We may feel something, but we can't see what it is. There are attempts using IR and raman spectroscopy, for instance; they are at a stage where researchers try things but it's actually difficult to handle and have several limitations. However, something like this would help in getting more information beside topographical and mechanical information.
For instance, it would be a real large improvement when we could do a scan and find out that something is stiff showing a high protein content and that the soft area could be related to lipids. Also recording the membrane potential simultaneously with topography, mechanics and chemical characterization would be a very useful improvement for all life cell research.
Where can readers find more information?
About Prof. Hermann Schillers

Prof. Dr. Hermann Schillers is Group leader at the Institute of Physiology II, University of Münster. He holds a Doctorate in Chemistry and a Habilitation from the University of Münster.
In 2003 he held a patent for a method for detecting diseases that are associated with defects of cystic fibrosis transmembrane conductance regulator (CFTR) protein.
About Bruker Nano Surfaces and Metrology

Bruker’s suite of fluorescence microscopy systems provides a full range of solutions for life science researchers. Their multiphoton imaging systems provide the imaging depth, speed and resolution required for intravital imaging applications, and their confocal systems enable cell biologists to study function and structure using live-cell imaging at speeds and durations previously not possible. Bruker’s super-resolution microscopes are setting new standards with quantitative single molecule localization that allows for the direct investigation of the molecular positions and distribution of proteins within the cellular environment. And their Luxendo light-sheet microscopes, are revolutionizing long-term studies in developmental biology and investigation of dynamic processes in cell culture and small animal models.