Researchers have found that it was possible to detect abnormal prion protein in the skin of 23 people who died from Creutzfeldt-Jakob Disease (CJD). They also found that exposing mice to skin tissue taken from the CJD patients caused them to develop prion disease.
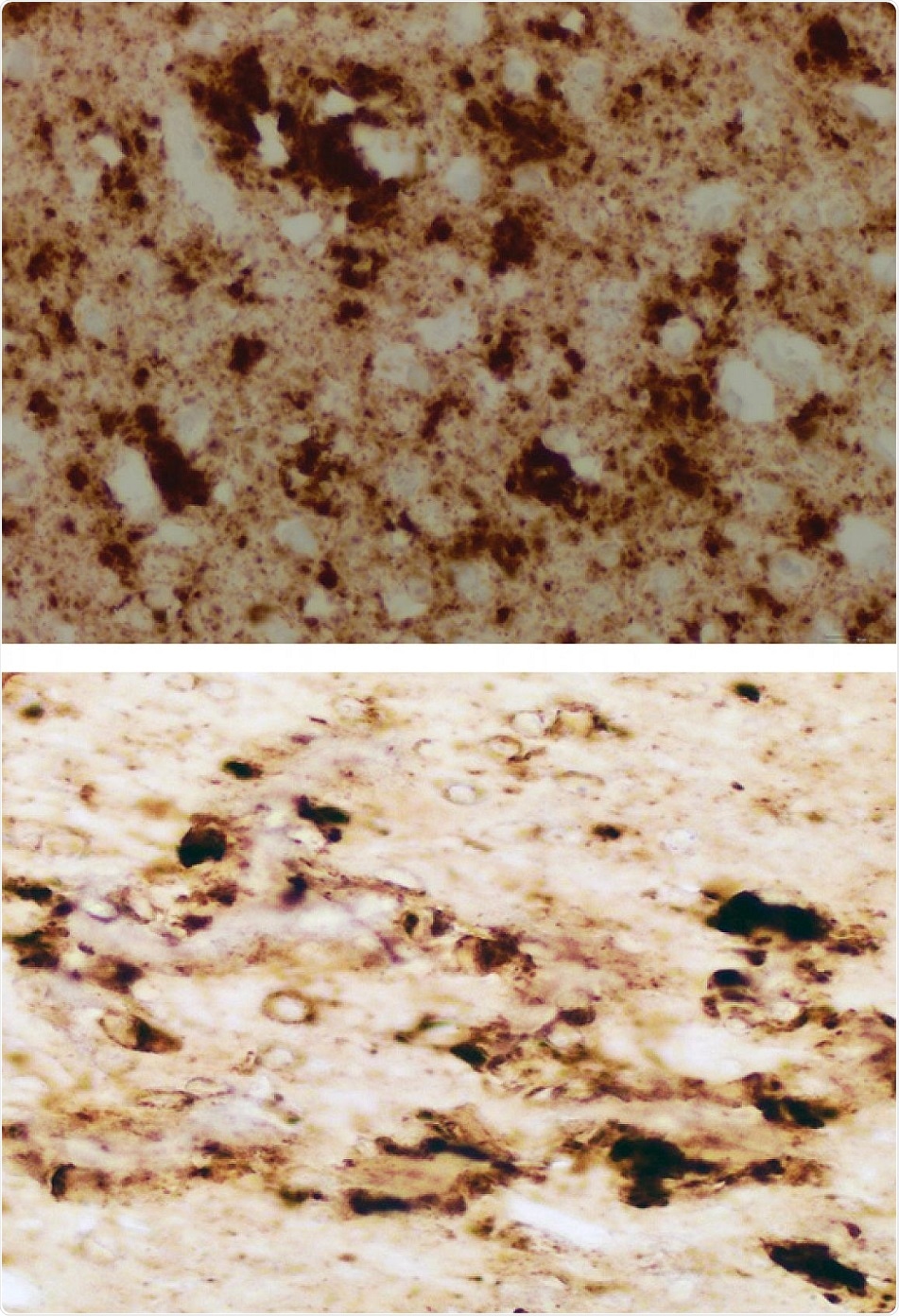
The brain of a patient who died from sporadic Creutzfeldt-Jacob disease (CJD) seems to be nearly identical to that of a mouse inoculated with the infectious prions extracted from the skin of patients who died from CJD. Credit: Case Western Reserve University
The study has raised questions about the possibility of prion diseases being transmitted during medical procedures that involve the skin, as well as the possibility of using skin samples to detect the diseases.
The study was conducted by scientists from the National Institute of Allergy and Infectious Diseases (NIAID) and various other collaborating groups.
Generally, people associate prion diseases with the brain, although it has been shown that clusters of the abnormal prion protein, which cause sponge-like holes in the brain, can accumulate in other organs including the liver, spleen, lungs and kidney. It is known that Sporadic CJD, which is the most common human prion disease, can be transmitted via invasive medical procedures involving the central nervous system and cornea, but transmission via the skin has not generally been considered a concern.
For the study, Byron Caughey, senior investigator at NIAID's Rocky Mountain Laboratories (RML) and colleagues used a prion disease test called Real-Time Quaking-Induced Conversion (RT-QuIC) to analyse skin extracts taken from 38 patients, 23 of whom who had died from CJD, and 15 of whom died from other causes. They also tested brain tissue taken from the CJD patients and from seven patients who had died from other causes.
As reported in Science Translational Medicine, the RT-QuIC test correctly identified the abnormal prion protein in all samples taken from the CJD patients and detected none in the patients who did not have CJD.
The team then inoculated 12 mice with brain tissue and 12 with skin tissue from two of the CJD patients and found that all of them developed prion disease, although it took approximately twice as long for the mice exposed to skin tissue to develop the disease.
The authors say the study should trigger discussion about the risk of surgical instruments becoming contaminated and the risk associated with procedures that involve CJD patients, as well as the possibility of using the RT-QuIC as a skin-based diagnostic test for prion diseases in both humans and animals
Our objective has always been to facilitate RT-QuIC testing using the most broadly available and least-invasive sample possible, whether that is blood, skin, nasal brushings, or other samples,"
Byron Caughey, Senior Investigator, NIAID's Rocky Mountain Laboratories
The team is continuing to develop RT-QuIC applications, including research into when and where the abnormal prion protein appears in the skin, as well as how its infectious forms can be inactivated.
Source: https://eurekalert.org/pub_releases/2017-11/nioa-nsa112217.php