Jan 4 2018
Scientists at Helmholtz Zentrum München have discovered a mechanism that amplifies the autoimmune reaction in an early stage of pancreatic islet autoimmunity prior to the progression to clinical type 1 diabetes. If the researchers blocked the corresponding molecules, the immune system was significantly less active. The study was conducted under the auspices of the German Center for Diabetes Research (DZD) and was published in the journal 'Science Translational Medicine'.
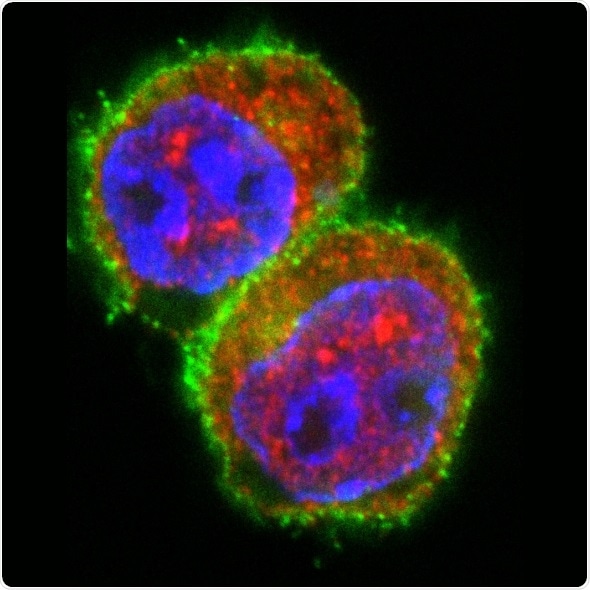
T cells after stimulation in the presence of a miRNA181a mimic. Immunofluorescent staining of the T cell marker CD4 (green), the transcription factor NFAT5 (red) and the nucleus (blue). The stimulation in the presence of a miRNA181a mimics results in an increase of NFAT5 expression in T cells. Source: Helmholtz Zentrum München
Type 1 diabetes is the most common metabolic disease in childhood and adolescence. In this disease, the body's own immune system attacks and destroys the insulin-producing cells of the pancreas. Regulatory T cells (Tregs) play an important role in this process: In healthy people, they suppress excessive immune reactions and thus prevent autoimmune diseases.
Dr. Carolin Daniel's team is investigating why Tregs fail to protect the islet cells in type 1 diabetes. She is a group leader at the Institute for Diabetes Research (IDF) of Helmholtz Zentrum München and a scientist in the DZD. In the current study, she and her team elucidated a mechanism that causes fewer Tregs to be produced during islet autoimmunity onset and that therefore allows the immune system to get out of control and attack.
According to the findings of the study, miRNA181a and NFAT5 molecules play a key role. "We showed that miRNA181a leads to the activation of the transcription factor NFAT5 during islet autoimmunity onset," said Daniel. "The consequence is an inhibition of Treg induction and thus increased immune activation."
Axis pharmacologically interrupted
In order to test the suitability of this new finding for possible therapeutic approaches, the scientists led by the first author Isabelle Serr investigated a preclinical model with early-stage islet autoimmunity. If the researchers interrupted the miRNA181a/NFAT5-axis, they observed a significantly lower activation of the immune system and an increased formation of Tregs. This was achieved both by the pharmacological inhibition of miRNA181a as well as of NFAT5.
"The targeted inhibition of miRNA181a or NFAT5 could open up new approaches to reduce the activity of the immune system against its own islet cells," said Professor Anette-Gabriele Ziegler, director of the IDF. "The combination with other immune modulating therapeutic approaches would also be conceivable as an intervention."
In the future, the scientists want to further investigate these findings in preclinical tests. To this end, humanized models will be used to test whether the combination of insulin vaccination and inhibition of the miRNA181a/NFAT5 axis leads to a more tolerant immune system towards insulin-producing cells.