Sponsored Content by PittconDec 17 2018
What is a mechanically interlocked molecule (MIM) and how have they advanced our understanding of chemistry?
A mechanically interlocked molecule is one that contains a mechanical bond. A mechanical bond is different from any previous type of bonding in chemistry as it involves a threading process of one molecular component through another.
There are two archetypical types of mechanically interlocked molecules. The first is called a catenane, and this is derived from the Latin word for chain, “catena”. The simplest example would be two interlocked rings.
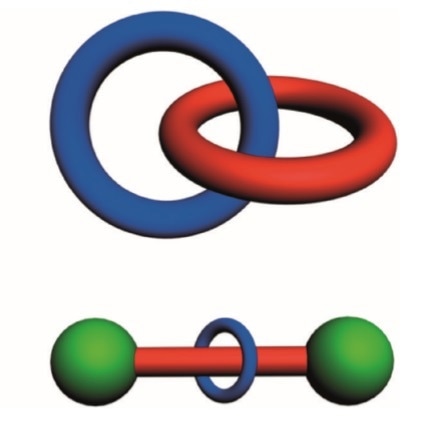
Graphical representations of a catenane and a rotaxane. © Stoddart, J. Fraser. "Mechanically interlocked molecules (MIMs)—Molecular shuttles, switches, and machines (Nobel Lecture)." Angewandte Chemie International Edition 56.37 (2017): 11094-11125.
Since the discovery catenane, our understanding of chemistry has developed with synthetic chemists like myself becoming more adventurous. We have made catenanes with three, four and even five rings - appropriately named the Olympiadane. At a very precise molecular level with all the necessary characterization, we got up as far as a seven catenane in the 1990s. Subsequently, the holy grail has become something called a polycatenane - a polymer with an endless number of interlocked rings.
How do molecules become mechanically interlocked?
The discovery of MIM’s was made efficient by the field of what has been called supramolecular chemistry, which means “beyond the molecule”. Here, instead of looking at a compound, you look at a complex - examining two molecules coming together and interacting with each other.
Of course, this concept is nothing new, and is in fact a broad field of science that is well-recorded in our biological receptor sites and, ultimately, in drugs that are introduced into our bodies to often interfere with these receptor sites. However, what scientists Jean-Marie Lehn and Donald Cram brought to it, was the fundamental aspect of doing it with artificial systems. Initially, these artificial systems were just based on large rings called crown ethers, introduced to our chemistry by a man named Charles Pedersen.
In 1987, the Nobel Foundation honored Pedersen, Cram, and Lehn for their invention of supramolecular chemistry, and this left us with an ability to not only bring molecular components together, but then also make sure they couldn't fly apart by putting them into the context of having a mechanical bond.
Please give an overview of your research involving the abilities of Rotaxane.
A type of mechanical machine which has overtaken catenanes in terms of applications is the rotaxane. This is based on a dumbbell at its simplest level - think of a weightlifter in the Olympic Games, lifting large weights that are on the end of a big rod. Then, minimally there would be one ring on this dumbbell on the axle. This ring, is to some extent, free to move along the dumbbell.
The exquisite feature that we noticed when looking at the movement of these molecular components, is that the rings in a catenane don't move in a circumrotational manner with respect to each other – instead, the rings have certain “sticky points” which they like to be around.
In '91, we arranged to have two sticky points along the axle of a dumbbell with one ring. Here, in solution, we were able to watch this ring jump between the two sticky points by various methods. With the particular design we had, it was jumping about 2,000 times a second and we named it a molecular shuttle. When we desymmetrized it, it gave us a molecular switch. Then, later on, we went on to make artificial molecular machines.
What is meant by the term “molecular machine”?
It’s rather difficult to define and there's not total agreement in the scientific community as to what it is, as for example, physicists look at machines a differently to chemists. If we look at the types of MIM’s that we have discussed, the main difference between going from a switch to a machine is that if you turn a switch on and switch it off, you do not have to do any work to the system.
Whereas with a machine, you have to demonstrate that in some shape or form you have done some work. You have moved components in a way that usually they would not wish to go without the supply of either a chemical fuel or light or electricity. It's a matter of feeding the molecules with some energy in order to, in some cases, raise the energy of the whole molecular system.
Molecular Machines for Drug Delivery
Since the discovery of the rotaxane, how have you used molecular machines in your work?
We've been working on molecular pumps where we take the rings and put them onto what I would call a pseudo-dumbbell, where one end of the dumbbell is not a fully-fledged stopper, a ring can slip over it and pass over the stopper. We can then devise a molecular pump on one side of the pseudo-dumbbell. Then, we bring a ring on to the pumping portion and pump it over what can be described as a “speed bump”, before putting it onto a collecting chain.
We’re hoping to pump rings onto common polymer chains where they would not normally be, so that as these rings get put on, and they're all carrying very high positive charges, they repel each other and you’re able to raise the energy of the system.
Pittcon recently announced that you will be presenting the Wallace. H. Coulter Lecture next year. What story will you be telling in your talk?
I expect that some people will attend my talk to hear about artificial molecular machines and the work leading up to that, so of course I will give an introduction much like I have described here, from 1985 up to the present day for the development of, first of all, the molecular recognition, then the making of the catenane, the rotaxane, the molecular shuttle, molecular switch, on to the molecular motor.
However, I felt that in an environment such as Pittcon, a meeting where the latest advances and discoveries in analytical chemistry are discussed, it was appropriate to talk about some examples of work that has emerged in our laboratory quite serendipitously, one in 2010 and the second in 2013. Therefore, the title I've chosen is, "Serendipity Stokes Discovery: Disrupting Established Industries”.
The two stories that I will tell are around compounds called cyclodextrins. A cyclodextrin is a cyclic molecule that is obtained from starch. In the case of this glucose-based polymer, a microorganism is capable of breaking the polymer chain and then fixing them into a circle/doughnut.
Our discovery in 2010 was fascinating to me, as we found that a cyclodextrin with eight sugar units, which has eight-fold symmetry but also commutes with that four-fold symmetry, did something that was quite unexpected in the presence potassium ions. Six of these doughnuts go to form a cube, linked by potassium ions, then these cubes are all linked together in an extended framework that goes on for the size of the cubic crystal, which can be certainly millimeters, if not almost a cubic centimeter in size.
This equates with a field of chemistry that has been very much developed this last 20 years called metal-organic frameworks (MOFs). I am quite happy to claim that this is the only MOF that is benign, green, edible, and ready to be used in all kinds of applications. In 2014, it led to the registration of a company called PanaceaNano, under the leadership of Youssry Botros, who recently received the Chicago Innovation Award for 2018.
What do you think the future holds for the use of the cyclodextrine MOF?
The product that Youssry decided to go for shocked me initially. I asked him, why cosmetics?
Now, I realise that this is a huge market worldwide and it's one that has a great deal of value added to it. To put it simply, even though our cyclodextrin is relatively inexpensive, in very few steps, we can make something that is quite revolutionary when it comes to the world of cosmetics. The revolution is that you have this very highly porous structure.
Going forwards, we’re also looking at potentially exploring the safe supplement market.
Overall, it's very exciting to me. It's one thing to win a Nobel prize, it's another thing to have the prospect of being able to see a company maybe come to fruition.
What other cyclodextrin applications will you be discussing at Pittcon?
The second serendipitous discovery involves a six-unit cyclodextrin. Again, potassium ions are involved and represent the positive ion. The negative counter ion is tetrabromoaurat. This is a gold atom surrounded by four bromines, and it is negatively charged. If you add this salt to alpha-cyclodextrin in solution, within minutes, needles containing the gold salt are formed and precipitate out. So, in summary, we have a new way of isolating gold from gold mines.
For the last 150 years or so, sodium cyanide is mainly used for gold isolation in gold mines. This is a nasty, and non-environmentally friendly chemical. I’m optimistic that in time, there will be a strong drive towards having environmentally friendly ways of isolating gold, and that this solution could be our cyclodextrin-based process.
Why do you enjoy coming to events like Pittcon to share your story? What would you like people to take home from your talk?
There are many things going on in our laboratory and what I want people to take away from the stories I share at Pittcon, is that it's not so much about me or my research; it's about the many spectacular students that help me with my work in the lab, and also develop their own opportunities.
There's a group of about 30 mainly postdoctoral researchers and they have a huge amount of freedom. I essentially say to them, "You’re not going to see much of Fraser Stoddart, but you've got the opportunity to come here where there is good financial resource, there is a first-rate intellectual environment, and you have an opportunity to launch your own career in research". I feel truly honored and inspired to experience a culture of practicing my hobby with bright young people.
What does the future hold for your research?
Ultimately, I would like to be able form the Stoddart Foundation and have a means of putting a lot of money back into science in the way that I think it should be that is in support of blue skies research.
Where can readers find more information?
About Sir Fraser Stoddart

The academic career of Fraser Stoddart can be traced through thick and thin from the Athens of the North to the Windy City beside Lake Michigan with interludes on the edge of the Canadian Shield beside Lake Ontario, in the Socialist Republic of South Yorkshire, on the Plains of Cheshire beside the Wirral, in the Midlands of the Heartland of Albion, and in the City of the Angels beside the Peaceful Sea. He has been a member of the faculty at Northwestern University since 2008. He is a Board of Trustees Professor and Director of the Center for the Chemistry of Integrated Systems.
Sir Fraser Stoddart has published over 1,150 scientific papers and was awarded the Nobel Prize in Chemistry in 2016, ‘for the design and synthesis of molecular machines’. His research interests are in chemistry beyond the molecule, which, combined with his interest in templation, has led to the template-directed synthesis, based on molecular recognition and self-assembly processes, of a wide range of mechanically interlocked molecules, bistable variants of which have found their way in the form of switches into molecular electronic devices and drug delivery systems. In terms of molecular structure, his research straddles the size regime from the mesomolecular scale all the way up to the nanoscopic, microscopic and macroscopic levels: it includes wholly synthetic polymers and metal-organic frameworks. He also embraces radical chemistry in both the supramolecular and mechanostereochemical domains.
About Pittcon
 Pittcon® is a registered trademark of The Pittsburgh Conference on Analytical Chemistry and Applied Spectroscopy, a Pennsylvania non-profit organization. Co-sponsored by the Spectroscopy Society of Pittsburgh and the Society for Analytical Chemists of Pittsburgh, Pittcon is the premier annual conference and exposition on laboratory science.
Pittcon® is a registered trademark of The Pittsburgh Conference on Analytical Chemistry and Applied Spectroscopy, a Pennsylvania non-profit organization. Co-sponsored by the Spectroscopy Society of Pittsburgh and the Society for Analytical Chemists of Pittsburgh, Pittcon is the premier annual conference and exposition on laboratory science.
Proceeds from Pittcon fund science education and outreach at all levels, kindergarten through adult. Pittcon donates more than a million dollars a year to provide financial and administrative support for various science outreach activities including science equipment grants, research grants, scholarships and internships for students, awards to teachers and professors, and grants to public science centers, libraries and museums.
Visit pittcon.org for more information.