Researchers from the Washington University School of Medicine have developed an implantable device that could one day help people with bladder problems, without the need for electronic stimulators or medication.
 Magic Mine | Shutterstock
Magic Mine | Shutterstock
As reported in the journal Nature, the tiny soft device detects overactivity in the bladder and uses light from integrated LEDs to reduce the urge to urinate.
The researchers found that the device worked in laboratory rats, but they hope it could one day be developed to help humans with overactive bladder for whom incontinence, pain, burning or a frequent urge to urinate are often sources of distress.
Many people with such bladder problems are currently treated with electronic stimulators that control a nerve in the bladder to improve overactivity.
Senior investigator Dr. Robert Gereau says that although there is a benefit to that sort of nerve stimulation, there are also some off-target side effects that result from a lack of specificity with those older devices. They can disrupt nerve signaling in other organs, for example.
The researchers hope their new implantable device will bypass such side effects.
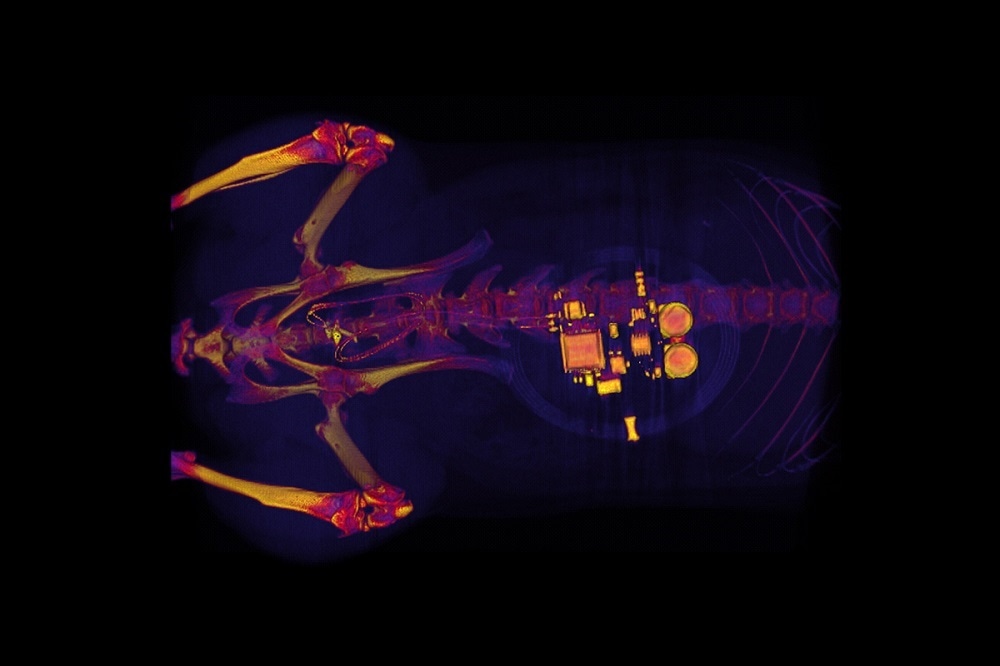
Using a minor surgical procedure, the soft, stretchy device is fitted around the bladder, where it then expands and contracts as the organ fills and empties.
Proteins called opsins are injected into the bladder and carried by a virus that binds to neurons in the organ, making those cells light sensitive. Those nerve cells can then be activated using optogenetics – the control of cell behavior through light.
Bluetooth communication enables the team to read real-time information and a simple algorithm is used to see whether the bladder is full or empty and when it is emptying too frequently.
 Gereau Lab | Washington University
Gereau Lab | Washington University
The researchers believe a similar device could be used to treat people, although those devices would be larger and could be implanted using catheters rather than surgery.
"We're excited about these results. This example brings together the key elements of an autonomous, implantable system that can operate in synchrony with the body to improve health," said Dr. John Rogers, co-senior author.
The team also thinks the approach could be used to treat other conditions, such as chronic pain or to stimulate pancreatic cells to secrete insulin, for example.
However, one potential obstacle is using the viruses to bind light-sensitive proteins to cells in organs.
We don't yet know whether we can achieve stable expression of the opsins using the viral approach and, more importantly, whether this will be safe over the long term. That issue needs to be tested in preclinical models and early clinical trials to make sure the strategy is completely safe."
Dr. Robert Gereau, Co-senior Author