Plants have many superpowers, such as manufacturing compounds to help repel pests, protecting themselves from environmental hazards, and providing a plethora of essential oils and other compounds that can heal a multitude of infections and other diseases.
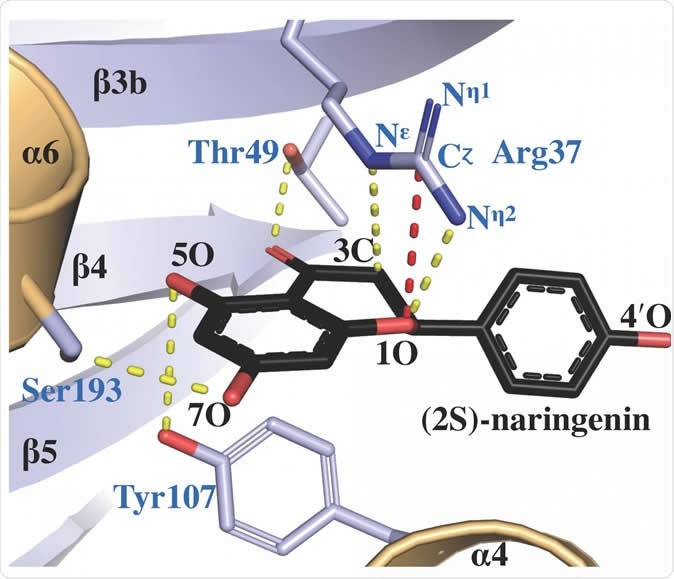
The protein X-ray crystal structure of chalcone isomerase, complexed with a product molecule called (2S)-naringenin, reveals how the active site arginine (labeled as Arg 37) facilitates catalysis of the correct isomer. Image Credit: Salk Institute/ACS Catalysis
Now, adding up to the plant’s superpowers, a team of researchers at the Salk Institute, studied how plants evolved their abilities to manufacture natural chemicals. They have found a new enzyme in plants, dubbed as chalcone isomerase, that has evolved to help plants create products essential for their survival.
The team hopes that the new information they’ve uncovered can help in formulating new products that can benefit humans, including improved crops and some medications to cure diseases.
“Since land plants first appeared on earth approximately 450 million years ago, they have developed a sophisticated metabolic system to transform carbon dioxide from the atmosphere into a myriad of natural chemicals in their roots, shoots and seeds,” Professor Joseph Noel, lead author of the study, said.
“This is the culmination of work we’ve been doing in my lab for the past 20 years, trying to understand plant chemical evolution. It gives us detailed knowledge about how plants have developed this unique ability to make some very unusual but important molecules,” he added.
What is chalcone isomerase?
Chalcone isomerase, also known as chalcone-flavanone isomerase, is a plant enzyme responsible for the isomerization of chalcone to naringenin, which is a vital step in flavonoid biosynthesis.
Flavonoids are a large group of natural products that exist in the flowers, leaves, seeds, and fruits of many plants. These natural chemicals play pivotal roles in the various facets of plant development in response to stress, including auxin transport, resistance to pathogens, and ultraviolet light protection.
For humans, flavonoids have many proven benefits to health, like antimicrobial, anticancer, and antioxidant properties, and may help in reducing the risk of several chronic diseases, such as obesity, cardiovascular disease, and diabetes.
Aside from that, chalcone isomerase also acts as a catalyst to accelerate chemical reactions in plants, including ensuring that the chemicals manufactured in the plant are in their proper forms.
Implication to human health
In formulating and manufacturing drugs, it’s essential that the medicines are created precisely, with the correct version or isomer, because using the wrong one can lead to adverse effects. If scientists study how chalcone isomers work, they can apply what they learn to accelerate the production of correct isomers of drugs and other products, adding to more effective therapies beneficial to human health.
In the study, the researchers utilized many structural biology techniques to study the unique shape in the enzyme. Also, they explored how the enzyme’s shape changes while it was interacting with other molecules. During the experiments, the team has identified the exact part of the enzyme’s structure to enable it to catalyze reactions effectively and rapidly, while making sure it makes proper and accurate isomer.
The reactions induced by chalcone isomerase stimulate a series of plant activities, one of which is the conversion of metabolites into flavonoids, which are phytonutrients proven to provide a wide range of health benefits.
Key answer to a long-standing question
The understanding of chalcone isomerase provide new information, showing how nature has solved a problem that chemists and scientists have been looking for a long time.
The researchers performed structural studies and computer modelling and discovered that arginine, an amino acid, linked to chalcone isomerase and allowed it to play the major role in how the enzyme’s reactions were catalyzed.
They found that the accurate locations of arginine in the active site of the enzyme when the reactions occurred. Without arginine, it won’t work. Hence, the scientists conclude that understanding the activities of chalcone isomerase can help chemists in studying plants.
“It’s absolutely vital to have this kind of foundational knowledge to be able to design molecular systems that can carry out a particular task even in the next generation of nutritionally dense crops capable of transforming the greenhouse gas carbon dioxide into molecules essential for life,” Noel explained.
Journal reference:
Burke, J., La Clair, J., Philippe, R., Pabis, A., Corbella, M., Jez, J., Cortina, G., Kaltenbach, M., Bowman, M., Louie, G., Woods, K., Nelson, A., Tawfik, D., Kamerlin, S., and Noel, J. (2019). Bifunctional Substrate Activation via an Arginine Residue Drives Catalysis in Chalcone Isomerases. ACS Catalysis. https://pubs.acs.org/doi/10.1021/acscatal.9b01926