Prior research by the same team showed that electromagnetic waves in the radiofrequency spectrum was protective against memory loss in young mice with AD and reversed memory loss in older AD mice.
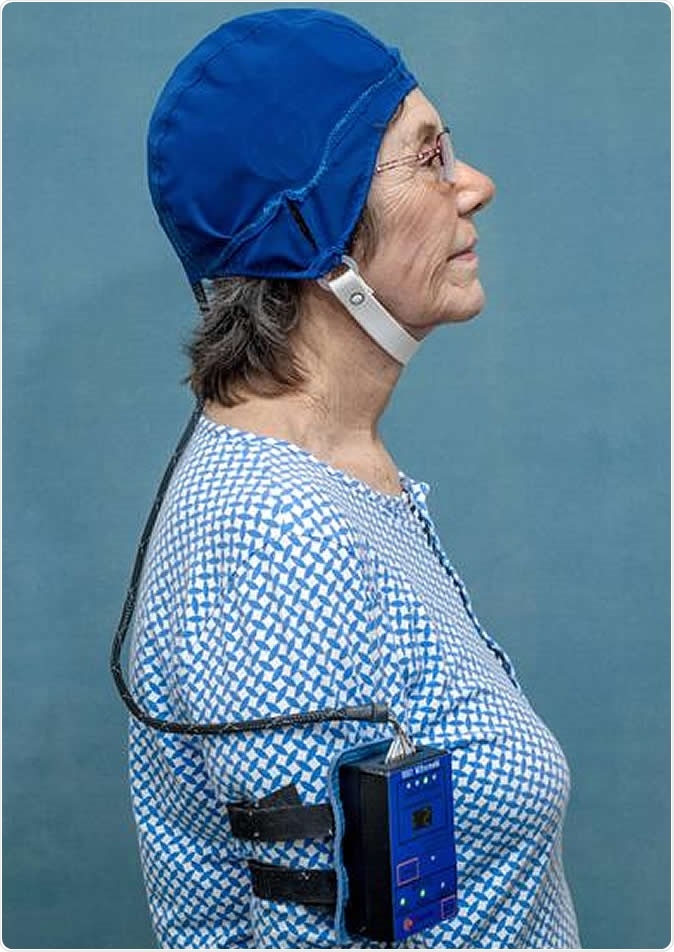
Patient wearing MemorEM. Credit: NeuroEM Therapeutics, Inc.
The study and its outcomes
In the current study they carried their research forward by using Transcranial Electromagnetic Treatment (TEMT) two times a day in one-hour sessions, applied by a pioneering MemorEM™ head device. The new device comprises a head cap that contains an array of extremely specialized electromagnetic wave emitters. These are designed to emit EM in sequence, and the therapy is easily do-able by caregivers at home. Moreover, there is almost no restraint on mobility for the duration of the treatment, so that the patient can do almost anything around the house during this time.
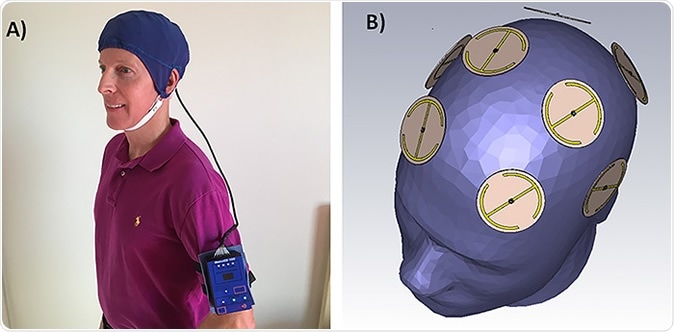
The initial results showed the safety of the wearable electromagnetic (EM) wave-emitting headgear, which was tested in 8 patients, all aged 63 years or more, with mild to moderate AD. There was no abnormal behavior, or change in body functioning. Following the treatment, brain scanning was done to rule out small tumors, or microscopic brain hemorrhages. The brain temperature during long-term TEMT in mice was recorded and showed no significant heating effect, which is again an important safety indicator. Many large studies have confirmed that exposure to an EM field at power levels and frequencies comparable to those used by the MemorEM do not induce cancer.
The tests also showed that their ADAS-cog score, which assesses their memory, thinking, judgment and other cognitive skills, was raised by 4 points or more in 7/8 patients. This score is used as the gold standard for judging the efficacy of all AD treatments, and the four-point increase shows a large increase in cognitive performance which translates into clinically important improvement in mental function. This is all the more satisfying when it is contrasted with the typical 4+ decline in this score over one year in patients with AD. In other words, it could be interpreted as turning the clock back one whole year on the disease – a significant improvement in cognitive performance.
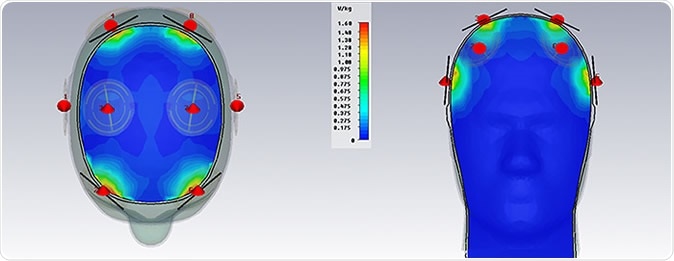
Other cognitive tasks that were tested include the Rey AVLT task which looks for word recall; the treatment produced a clinically significant increase in this parameter both immediately after treatment and two weeks thereafter. These patients also demonstrated a 50% reduction in memory loss. None of these results could be attributed to familiarity with the tests on repeated performance.
Laboratory testing was also performed to analyze the level of AD markers, in both blood and cerebrospinal fluid (CSF). The levels of these markers after the treatment were altered from their baseline (pretreatment) concentrations just as expected if the tau and beta-amyloid proteins were really being broken up by the TEMT.
Brain scanning using magnetic resonance imaging (MRI) showed that the brains of these patients mirrored better interneuronal communication in the cingulate cortex, a brain area that is deeply involved in cognitive integration.
The improvement was not only very marked but sustained, being present even when the patients were reassessed two weeks after the end of treatment. This led the investigators to weigh the hypothesis that, in the words of researcher Gary Arendash, “the Alzheimer's Disease process itself was being affected.”
How does it work?
While the investigators are not completely sure, they think that the TEMT can penetrate the brain neurons and break up the clustered soluble aggregates of toxic amyloid-beta and tau proteins inside the cells that cause AD. The intracellular tau and beta-amyloid proteins act in multiple ways to produce AD: mitochondrial damage leading to nonproduction of ATP, the body’s energy molecule; destroying the microtubules that are essential to cell structure and transport; loss of the neuronal dendrites which receive information from other neurons; and loss of synaptic function. Therefore, the disaggregation of both amyloid and tau oligomers is the key change to be targeted in order to protect the brain against memory loss and also to reverse cognitive impairment that has already occurred.
TEMT was also associated with increased mitochondrial activity, and increased neuronal activity – a combination benefit that has so far been unheard of in drug-based treatment of AD.
In this way TEMT is superior to all existing therapies undergoing clinical trials. All existing drugs find much difficulty both in passing the blood-brain barrier and in entering brain cells. In addition, the few that do cannot fight these protein aggregates, targeting either the extracellular beta-amyloid plaques or intracellular fibrillary tau tangles – though these are not the real culprits in AD. Other non-drug approaches such as deep brain stimulation or transcranial magnetic stimulation have not produced significant cognitive benefits.
All the patients wanted to keep using the device; they are now participating in an ongoing extension of the study which will last, on average, 17 months.
All in all, the investigators think they have good cause to celebrate; this revolutionary approach to treating AD could lead to a successful outcome where all previous drug-dominated approaches have failed.
The next step for NeuroEM Therapeutics is a clinical trial in about 150 people with mild to moderate AD. The subjects are being recruited and the trial is set to begin later this year. If this important multicenter trial confirms device safety and improved cognitive function, the company will apply for FDA approval of the MemorEM as a therapy for AD.
Journal reference:
A clinical trial of transcranial electromagnetic treatment in Alzheimer’s disease: cognitive enhancement and associated changes in cerebrospinal fluid, blood, and brain imaging. Gary Arendash, Chuanhai Cao, Haitham Abulaban, Rob Baranowski, Gary Wisniewski, Lino Becerra, Ross Andel, Xiaoyang Lin, Xiaolin Zhang, David Wittwer, Jay Moulton, John Arrington, & Amanda Smith. Journal of Alzheimer's Disease, vol. 71, no. 1, pp. 57-82, 2019. DOI: 10.3233/JAD-190367. https://content.iospress.com/articles/journal-of-alzheimers-disease/jad190367