Scientists at Newcastle University in the UK have developed a new test that can more accurately predict whether skin cancer is likely to return.
The researchers say that for patients with the earliest stages of melanoma, the test can reliably predict whether the cancer is likely to spread to other parts of the body or recur once it has been removed.
The team hopes the pioneering test will be available for use within two years.
 Pop Paul-Catalin | Shutterstock
Pop Paul-Catalin | Shutterstock
Melanoma prevalence is on the rise
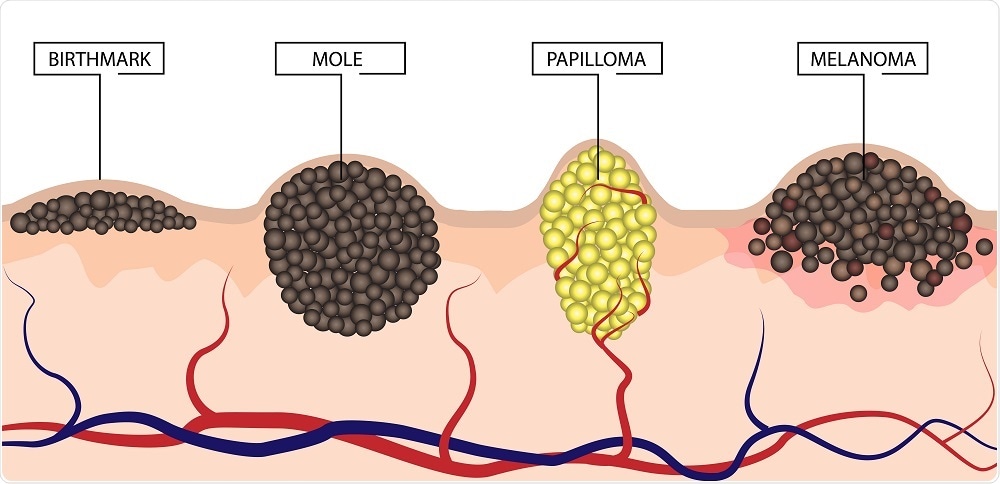
The prevalence of melanoma is increasing worldwide, with an estimated 17,000 people diagnosed with the condition every year in the UK alone. Melanoma is the deadliest form of cancer, but if it is diagnosed early, surgical removal is usually sufficient to eliminate it. However, in some cases, the cancer can return.
Currently, biopsies taken from removed tumors are studied by pathologists under the microscope to determine the stage of the disease and the risk of it spreading.
Patients defined as low risk are followed up in the clinic for as long as five years and these are the patients that the new test is able to identify. Around 10% of patients with stage 1 disease will develop metastasis, which has a poor prognosis and a 5-year survival rate of just 15 – 20%.
The developers of the new test say the current staging criteria for melanoma remain unable to identify high‐risk stage I tumor subsets. They are unable to reliably identify which individuals with seemingly low‐risk, early melanomas are at specific risk of disease progression.
AMBLor: The new test
The test, which is called AMBLor, provides stage 1 melanoma patients with more accurate information about their risk of the cancer progressing and spreading.
As a patient, the AMBLor test tells you if you're in the low risk category - and can offer you reassurance. It could also save the NHS up to £38 million a year by reducing the number of follow-up appointments for those identified as low-risk."
Penny Lovat, Chief Scientist
As reported in the British Journal of Dermatology, the researchers showed that two protein biomarkers, AMBRA1, and loricrin, which are usually present in the epidermis, are lost in patients in patients with the kind of early-stage melanoma that is associated with high-risk tumors. Among people with genuinely low-risk tumors, on the other hand, the markers are retained.
They then used this discovery to develop the AMBLor testing kit, which could potentially be applied to tumor biopsies to help doctors flag up patients with low-risk, less aggressive cancers.
“Building on our previous studies, this new research demonstrates that the loss or reduction of these proteins indicate that the tumor is more likely to spread allowing us to develop our test, called AMBLor. This can be applied to the standard biopsy and identifies those who have these low-risk, less aggressive cancers,” says Lovat.
 LiliiaKyrylenko | Shutterstock
LiliiaKyrylenko | Shutterstock
The test will benefit both low- and high-risk patients
Co-author Rob Ellis says that if somebody is identified as low risk, it may be that they do not need to attend the clinic as frequently as these patients do currently. Equally, if somebody is identified as higher risk, they could perhaps be offered different tests to investigate them further and follow them up more closely in the future.
“But also from the patients' point of view, apart from the physical health problems with the tumors there's a lot of psychological problems and being able to more surely reassure somebody ... puts patients' minds at ease,” he adds.
The team applied the test to 400 archived biopsies taken from patients who had stage 1 melanoma and showed that it could predict long term prognosis of the disease and potentially help doctors develop personalized treatment plans for patients.
What we have developed is a test which will offer personalized, prognostic information – so we will be able to more accurately predict if your skin cancer is unlikely to spread. This is a really exciting finding for clinicians and in the future, it will help us tailor the treatment and follow up appointments in an appropriate fashion.”
Rob Ellis, Co-author
Chairman of national charity congratulates the researchers
The study was funded by Cancer Research UK, The British Skin Foundation, The Newcastle Healthcare Charities, The North Eastern Skin Research Fund and the charity Melanoma Focus.
The chairman of Melanoma Focus, Paul Lorigan, has congratulated the researchers on their discovery, saying: “It offers the prospect of treating patients more accurately, reducing their stress and saving the NHS a great deal of money. Melanoma Focus is delighted to have helped fund this research.”
Lovat and colleagues are now seeking approval for the test to be made available to patients within a couple of years.
We are working with NICE to show the value of this test to the NHS and we are currently gathering evidence to present to them. We expect this to take less than 2 years."
Rob Ellis, Co-author