In a study recently published in Nature Communications scientists at Okayama University describe the detailed molecular pathogenesis of muscular dystrophy-associated cardiomyopathy in mice lacking the fukutin gene (Fktn), the causative gene for Fukuyama muscular dystrophy.
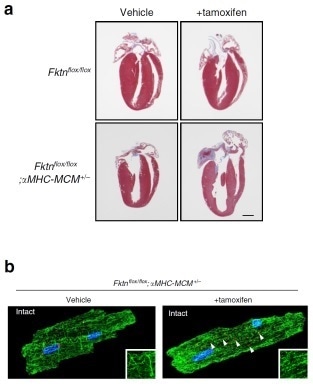
(A) Structure of the heart in healthy (top panel) and fukutin-deficient (bottom panel) mice in the presence or absence of tamoxifen, the elimination of fukutin gene-inducing drug. (B)Tamoxifen treatment resulted in microtubule clumping (green) and golgi body fragmentation (red) in fukutin-deficient mice.
Heart failure is the major cause of death for muscular dystrophy patients; however little is known for the molecular mechanism of muscular dystrophy-associated cardiomyopathy. In this study, a research team spearheaded by senior lecturer Katanosaka Yuki at Okayama University demonstrate for the first time the cellular and molecular pathomechanisms of muscular dystrophy-associated cardiomyopathy using mouse models of Fukuyama muscular dystrophy with a deficiency for the fukutin gene (Fktn- dystroglycans (DG).a), which encodes a Golgi-based ribitol-phosphate transferase that catalyzes the biosynthesis of tandem ribitol-phosphate structure on As DG and proteins of the dystrophin–glycoprotein complex provide structural support for the sarcolemma in muscle tissue, a loss of membrane fragility was thought to be a cause for cardiac dysfunction in these diseases collectively known as a-DGpathies. However, their data show that cardiac dysfunction in muscular dystrophy-associated cardiomyopathy occurs at the cellular cardiomyocyte level.
In this study, although cardiac Fktn -DG glycosylation and dystrophin-glycoprotein complex proteins in sarcolemma at all developmental stages, cardiac dysfunction was observed only in later adulthood, suggesting that membrane fragility is not the sole etiology of cardiac dysfunction. Younger Fktn-deficient mice show a vulnerability to hemodynamic stress conditions via impaired compensative hypertrophic response of cardiomyocytes. Adult Fktn-deficient mice exhibit altered cardiac morphology and dysfunction, suggesting that FKTN is critical for maintaining contractile function of individual cardiomyocytes.
In addition, the team show that acute Fktn-elimination causes the disordered Golgi-microtubule network in myocytes. Finally, the team show that treatment with colchicine (an FDA-approved drug for the treatment of familial Mediterranean fever) improved cardiac dysfunction of Fktn-deficient hearts via the recovery of myocyte shortening, which may open a new avenue for therapeutic strategies.
Background
Muscular dystrophy and heart failure: Muscular dystrophy is a genetic condition that results in debilitating muscle weakness from childhood. Symptoms range from difficulty with walking and running, frequent falls, and muscle stiffness to extreme pain. Although there are various types of muscular dystrophy, Fukuyama Congenital Muscular Dystrophy (FCMD), the second most prevalent in Japan, typically leads to cardiac and neurologic issues. FMCD results from a genetic mutation leading to loss of the fukutin protein.
Heart muscles: The heart muscles make up the walls of the heart and are responsible for it beating rhythmically. Damage to these muscles can result in heart attacks, arrythmias (irregular heartbeats), and heart failure. Proper myocyte structure and intracellular Ca2+ handling are important factors for healthy hearts. Microtubule are filaments required for the myocytes proper structure. The disorganization of microtubule induces the altered Ca2+ homeostasis in myocytes. In turn, golgi bodies are required for maintenance of the microtubule structure.
Source:
Journal reference:
Ujihara, Y., et al. (2020) Elimination of fukutin reveals cellular and molecular pathomechanisms in muscular dystrophy-associated heart failure. Nature Communications. doi.org/10.1038/s41467-019-13623-2.