Although severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is unlikely to infect human placenta through the canonical cell entry mediators, Wayne State University researchers showed that other interacting proteins may still play an important role during the viral infection. The study is currently available on the preprint server bioRxiv*.
SARS-CoV-2, a causative agent of the ongoing coronavirus disease (COVID-19) pandemic, enters the human cell by utilizing two proteins: a receptor known as angiotensin-converting enzyme 2 (ACE2) which aids in viral cell attachment, and an enzyme known as type II transmembrane serine protease (TMPRSS2) which further facilitates cell infection.
SARS-CoV-2 viruses are binding to ACE-2 receptors on a human cell, the initial stage of COVID-19 infection. Conceptual 3D illustration credit: Kateryna Kon / Shutterstock

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Pregnant women and their fetuses were considered a high-risk population from the start of the COVID-19 outbreak, as viral infections such as influenza, measles, varicella, Ebola, and Zika show increased severity in this delicate physiological state.
In addition, other coronaviruses (such as original SARS and MERS-CoV) have severe consequences for both mother and child, albeit vertical transmission has not been definitely proven (possibly due to only a handful of cases in these studies).
COVID-19 and pregnancy
But unlike the infections above, only about 15% of pregnant women test positive for SARS-CoV-2, and even those mostly have a mild symptomatic illness. Hence, the clinical specificities of pregnant women with COVID-19 are akin to those of non-pregnant adult individuals
Likewise, no unequivocal evidence of vertical transmission has been found. Children born to mothers with COVID-19 have negative tests for SARS-CoV-2, do not develop serious clinical presentation, and are swiftly discharged from the hospital.
Nonetheless, new evidence has appeared suggesting that the fetus can react to SARS-CoV-2 infection. This means that the expression of ACE2 and TMPRSS2 throughout pregnancy and in third-trimester chorioamniotic membranes may be of clinical significance.
Building upon their previous single-cell study of the placenta and RNA-sequencing data, this research question was tackled by the researchers from Wayne State University, University of Michigan in Ann Arbor, Michigan State University, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Detroit Medical Center and Florida International University.
Hunting viral receptors
Publicly available single-cell RNA-sequencing (scRNA-seq) data (alongside newly generated information) were used to appraise whether the receptors known to enable SARS-CoV-2 infection are expressed in the human placenta throughout pregnancy (including the decidual tissues).
This group of researchers has also evaluated the expression of SARS-CoV-2 receptors in the chorioamniotic membranes (which are known as the extraplacental membranes) in the third trimester, due to a potential role of these tissues as a point of entry for microorganisms invading the amniotic cavity and (possibly) the fetus.
Finally, this paper aimed to appraise whether the receptors for known congenital viruses infecting and crossing the placenta (primarily cytomegalovirus and Zika virus) can be detected using the pipeline in this study.
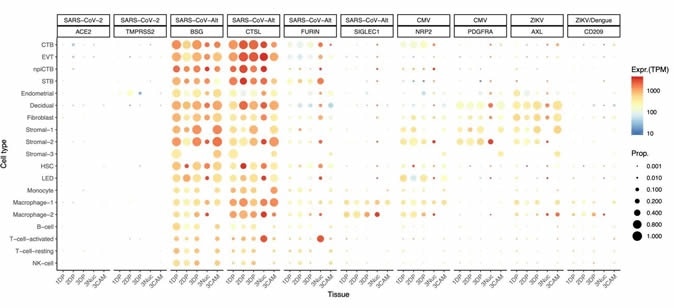
Dot plot depicting the expression of different viral receptors/molecules used by SARS-CoV-2, ZIKV, and CMV. Each row represents a different cell type, and columns are grouped first by virus type, receptor/molecule gene, and placental tissue/time-of sampling (1DP, 2DP, and 3DP represent the first, second, and third trimester, 3Nuc represents the third-trimester nuclei, and 3CAM represents the third-trimester chorioamniotic membranes). The size of the dot represents the proportion of cells that express the receptor with more than zero transcripts, and the color represents the average gene expression for the subset of cells expressing that gene in transcripts per million (TPM). Cell type abbreviations used are STB, Syncytiotrophoblast; EVT, Extravillous trophoblast; CTB, cytotrophoblast; HSC, hematopoietic stem cell; npiCTB, non-proliferative interstitial cytotrophoblast; LED, lymphoid endothelial decidual cell.
The lack of canonical cell entry mediators
Strikingly, the researchers found that only a handful of cells co-express ACE2 and TMPRSS2. By employing a rather permissive expression threshold of one transcript per cell, only four cells exhibiting co-expression were found in any of the three trimesters – resulting in an estimated count of less than 1 per 10,000 cells.
"Our results suggest that vertical transmission of SARS-CoV-2 is unlikely to occur unless facilitated by other concomitant pathological conditions resulting in a breach of the maternal-fetal crosstalk", report study authors.
However, there is a possibility that SARS-CoV-2 may infect the human placenta by utilizing alternative routes via interaction with other proteins. For example, in vitro studies have shown that Basigin (also known as CD147 or EMMPRIN) is a transmembrane protein that can provide an alternative cell entry for the virus when ACE2 and TMPRSS2 are not expressed.
Although placenta and chorioamniotic membranes are laden with this protein, this transcript is actually found in all human tissues and cell types. Therefore, other proteins may be necessary (such as cathepsin L and FURIN) to prime the SARS-CoV-2, and even various sialoadhesins may play a role in this process.
A low likelihood of vertical transmission
In conclusion, this novel single-cell transcriptomic analysis provides evidence that the placenta and fetus are unlikely to get infected by SARS-CoV-2 since its canonical receptor and protease (ACE2 and TMPRSS2, respectively) are minimally expressed by the human placenta.
"In addition, we showed that the SARS-CoV-2 receptors are not expressed by the chorioamniotic membranes in the third trimester", explain study authors. "However, viral receptors utilized by cytomegalovirus, Zika virus, and others are highly expressed by the human placental tissues," they add.
Albeit transcript levels are not always in correlation with protein expression, their findings indicate a low likelihood of placental infection and vertical transmission of SARS-CoV-2.
"However, it is still possible that the expression of these proteins is much higher in individuals with pregnancy complications related to the renin-angiotensin-aldosterone system, which can alter the expression of ACE2", caution study authors.
In any case, exact mechanisms and cellular receptors that could be exploited by SARS-CoV-2 are still under investigation. More extensive prospective studies are warranted to elucidate the possibility of vertical transmission in pregnancy completely.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Pique-Regi, R. et al. (2020). Does the human placenta express the canonical cell entry mediators for SARS-CoV-2? bioRxiv. https://doi.org/10.1101/2020.05.18.101485.
- Peer reviewed and published scientific report.
Pique-Regi, Roger, Roberto Romero, Adi L Tarca, Francesca Luca, Yi Xu, Adnan Alazizi, Yaozhu Leng, Chaur-Dong Hsu, and Nardhy Gomez-Lopez. 2020. “Does the Human Placenta Express the Canonical Cell Entry Mediators for SARS-CoV-2?” ELife 9 (July). https://doi.org/10.7554/elife.58716. https://elifesciences.org/articles/58716.