With many hundreds of thousands of deaths due to the current COVID-19 pandemic, there is a rush to find an effective vaccine or drug against the virus. A new study on the preprint server medRxiv* in May 2020 shows that the use of some biologically plausible drugs may be counterproductive, and care should be taken when trying out unproven therapies.
Early reports suggested that the acute and severe lung injury so characteristic of severe and critical COVID-19 illness was not due to viral-induced lesions but were the effect of a hyperactivated immune system. The resulting activation of multiple pro-inflammatory pathways, including IL-6 signaling, which in turn recruits other inflammatory cells and molecules, leads to acute respiratory distress syndrome (ARDS), coagulopathy, multi-organ dysfunction, and even death in a significant minority of cases.
The same kind of overactive immunity is seen in sepsis, where the primary injury to the body is not bacterial but is due to the overwhelming release of inflammatory mediators and toxic molecules. It is also a feature of the cytokine release syndrome (CRS) seen in some patients on CAR T-cell therapy. This has led to the use of the IL-6 blocker, tocilizumab, for immunomodulation.
Motivation for the Current Trial
The present use of tocilizumab is neither backed by evidence-based guidelines, nor are there any criteria on patient selection. It is important to note that suppressing natural pro-inflammatory chemicals like IL-6 can have harmful consequences, including impairment of immunity against viruses, bacteria, and fungi. In humans, tocilizumab treatment has been linked to severe bacterial infections of soft tissue.
The chance observation of some serious bacterial sepsis in critically ill COVID-19 patients treated with tocilizumab prompted the current trial to probe an association between the two.
How was Tocilizumab Tested?
The study included adults aged 18 years or over, admitted to the intensive care unit (ICU) between March 1 and April 27, 2020.
The selection of patients for analysis was randomized. Out of this group of 60 critically ill patients with COVID-19, tocilizumab 400 mg was given to 28 of them. Among these, three patients received an additional dose, but 1 of them got a single dose of 800 mg.
The researchers looked for the incidence of bacterial and fungal infection among the tocilizumab and no-tocilizumab groups. Baseline characteristics were similar in both groups. Both groups were also similar concerning the presence of immunosuppression and hypertension. Secondary bacterial infections were diagnosed by a high clinical suspicion, which led to the administration of antimicrobials.
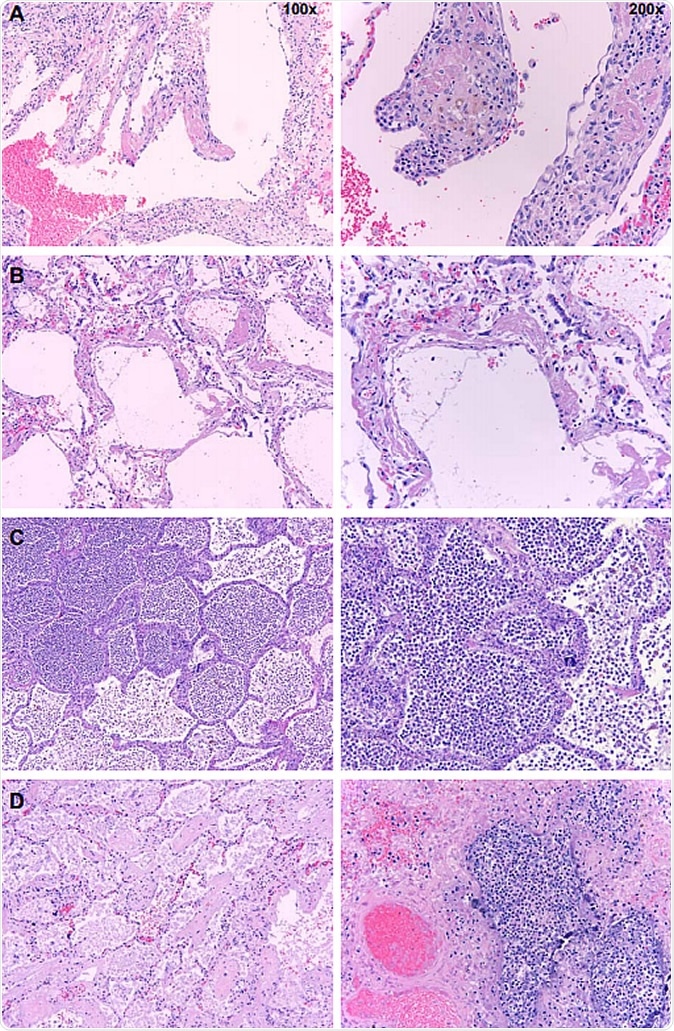
Postmortem histopathology of lungs from COVID-19 patients. Low (100x) and high power (200x) images of lungs from patients who died due to COVID-19. A. Organizing hyaline membranes are seen in the lung which has pre-existing emphysema (100x). Higher power shows fibrin, fibroblasts and mononuclear cells incorporated into the alveolar walls (200x). B. There is diffuse alveolar damage with hyaline membranes lining alveoli (100x). Higher power shows minimal inflammation with only a few mononuclear cells (200x). C. There is extensive intra-alveolar inflammation (neutrophils) in an otherwise normal lung (100x). On higher power, there is minimal alveolar wall thickening by inflammatory cells (also mainly neutrophils on myeloperoxidase staining and only rare lymphocytes) (200x). D. Majority of the sections from this case show organizing intra-alveolar fibrin (100x). Several foci of acute inflammation with alveolar filling are present, as seen here on higher power (200x).
Tocilizumab and the Risk of New Infections
The tocilizumab group had a higher rate of new bacterial infections compared to those who did not receive the drug at 64% and 31%, respectively. This included hospital-acquired pneumonia and ventilator-associated pneumonia.
When the risk of tocilizumab was assessed independently, its administration was found to carry an independent risk of secondary bacterial infection, the odds being almost fourfold in this group.
On autopsy, carried out on 7 patients, almost equally split between the tocilizumab and non-tocilizumab groups, all three in the first group showed signs of pneumonia, whereas two of the other four in the second group were patients with a history of stroke and dementia, with the lungs showing evidence of aspiration pneumonia. These two patients died on the day of admission, while the other two were admitted for 4 and 12 days. Their lungs showed diffuse injury but no signs of pneumonia.
Is Tocilizumab Safe in Ill Patients?
In light of this, it appears that limited evidence does not support the use of tocilizumab when a sick patient has an infection. In such a situation, the occurrence of secondary bacterial infection may cause increased occupancy of the ICU, as well as secondary fungal infections in a few patients.
Rather than routine use of tocilizumab or other immunomodulatory drugs, careful patient selection for such therapies should be the norm. This is made more difficult by the fact that there is no way to accurately and consistently identify those patients who are primarily suffering from the effects of a hyperimmune response and not a viral infection.
When microbial infection triggers the host immune response, it is as yet unproven that immunosuppressive therapy is beneficial. An earlier trial of anti-TNFα compounds in patients with sepsis drove up the death rate and increased the risk of infection. The current situation, therefore, underlines the need to carry out controlled trials rather than try out new drugs in a random, uncontrolled manner.
The researchers comment: “Our findings should raise physician awareness about the potential side effects of tocilizumab on pathogen clearance and development of secondary infections. It also calls for the urgent need to study all drugs including any immunosuppressive agents in randomized controlled trials, to better understand their role in any hyperimmune response and on clearance of SARS-CoV-2 and other hospital-acquired pathogens.”