The current pandemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that causes COVID-19 disease has caused 6.98 million cases and almost 400,000 deaths around the world, due in most cases, to terminal respiratory failure.
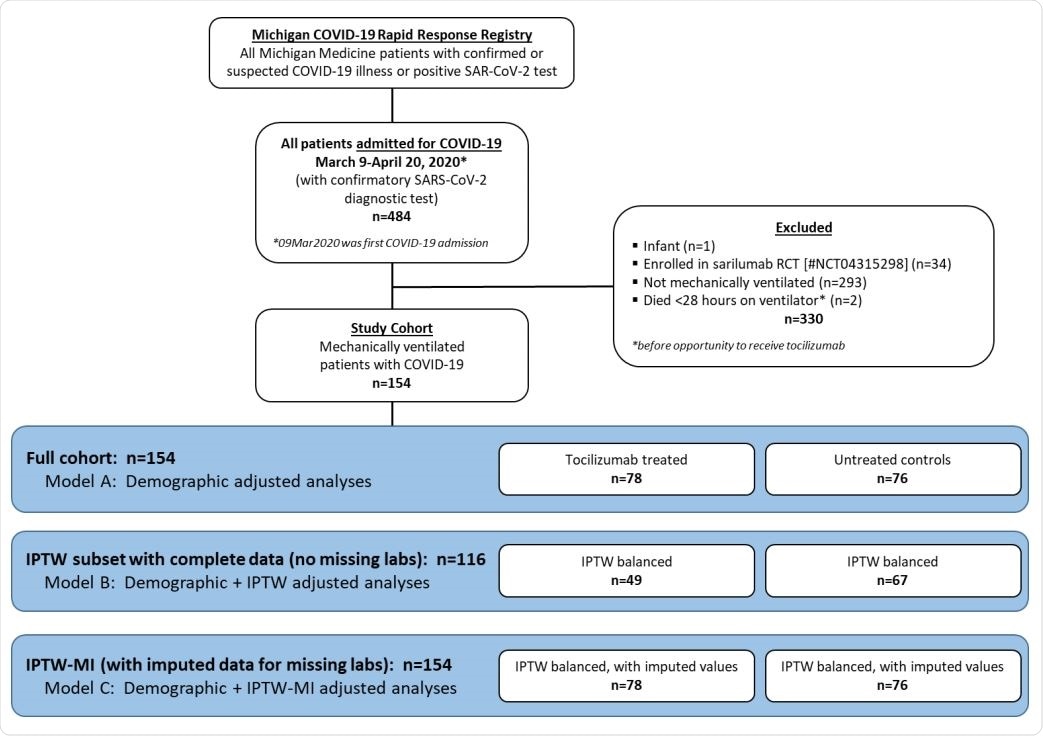
Study cohort flow chart

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Hyperinflammation in Fatal COVID-19 Disease
COVID-19 is a disease with a broad spectrum of manifestations, ranging from asymptomatic through mild cold-like symptoms to rapid decompensation, multi-organ failure, and acute respiratory distress syndrome (ARDS), leading to death in a significant percentage of cases (up to 20%). The underlying disease process in severe COVID-19 is thought to be hyperinflammation induced by the uncontrolled release of cytokines in response to the viral induction of the immune response.
The phenomenon is analogous to the cytokine release syndrome (CRS) seen in cancer patients treated with CAR-T cell therapy. One oft-seen finding in this condition is a high level of IL-6. At such concentrations, IL-6 can cause the lung epithelium to show hyperimmune responses, with overstimulation of macrophage recruitment and damage to the lung parenchyma. IL-6 could also activate other procoagulant pathways and cause endothelial injury as well, leading to thrombotic complications.
IL-6 blockers like tocilizumab have been found to be useful and are approved for the treatment of this condition.
On the other hand, IL-6 mediates beneficial T-cell responses, the cellular component of the adaptive immunity arm of the immune system. These cells develop a memory of the virus, prevent its reactivation, protect against superinfection, and help the lungs heal and remodel itself after an injury to restore normal function.
This has led to the proposal to use tocilizumab or sarilumab, which are IL-6 receptor antagonists, or IL-6 blockers like siltuximab, for the treatment of severe progressive COVID-19. While this has been used in several small studies, conclusive evidence of its efficacy is lacking. This could be due to poor reporting of study parameters, short follow-up duration, and lack of control groups. Moreover, tocilizumab can cause a blockade of all IL-6-dependent pathways, which could cause viral myocarditis and the presence of Candidemia.
The hospital protocol, therefore, decided on the use of tocilizumab in patients within 48 hours of being intubated or who were unable to maintain oxygen saturation with standard care. The dose chosen, at a single high dose of 8 mg/kg, was designed to saturate IL-6 receptors and thus quickly stop IL-6 signaling. In addition, it would hopefully minimize immunosuppression and promote rapid lung healing by allowing rapid clearance of the drug.
How Did the Researchers Assess the Utility of Tocilizumab?
The current study aimed to replicate this effect in the most critically ill COVID-19 patients, namely, those on mechanical ventilation. The outcome assessed was the probability of survival from the time of intubation. Patients who received this drug were evaluated against those who did not, using a multivariate regression model.
The trial included 154 patients with a positive RT-PCR for COVID-19, who were on mechanical ventilation for severe COVID-19 pneumonia. There were 78 on tocilizumab and 76 without this drug. The median duration of follow-up was 47 days. Both groups were similar with respect to baseline characteristics, but the mean age in the tocilizumab group was significantly younger, at 55, vs. 60 years in the other group.
This group was also noted to have a lower incidence of chronic lung disease at 10% vs. 28%, and chronic kidney disease, at 35% vs. 49%. The D-dimer values were, on average lower at the time of intubation at 2.4 vs. 6.5 mg/dL. Tocilizumab was administered mostly within 24 hours of intubation, but a quarter received the drug within 48 hours after intubation.
How Did Tocilizumab Benefit Patients?
The outcome showed that the use of tocilizumab was associated with a 45% reduction in the risk of death. Secondly, the status of the widely used 6-level ordinal outcome scale at 28 days was increased, with the odds improving by about 40% with each level of increase.
The number of patients discharged home over the study period was 56% vs. 40% in the tocilizumab and control groups, respectively, supporting the benefit of this drug. Moreover, it was noted that 47% of untreated patients remained on the ventilator at the end of the follow-up period, vs 18% of tocilizumab patients.
On the other hand, the drug was linked to an increased proportion of superinfections, at 54% vs. 26%. Overall, mechanically ventilated patients had a superinfection risk of 39%.
In the tocilizumab group, about 45% of these were ventilator-associated pneumonia, vs. 20% in the other group. In both groups, Staphylococcus aureus was responsible for approximately half of the cases of bacterial pneumonia.
Notwithstanding, the case fatality rate at 28 days was comparable among those with and without superinfection, at 22% vs. 15%.
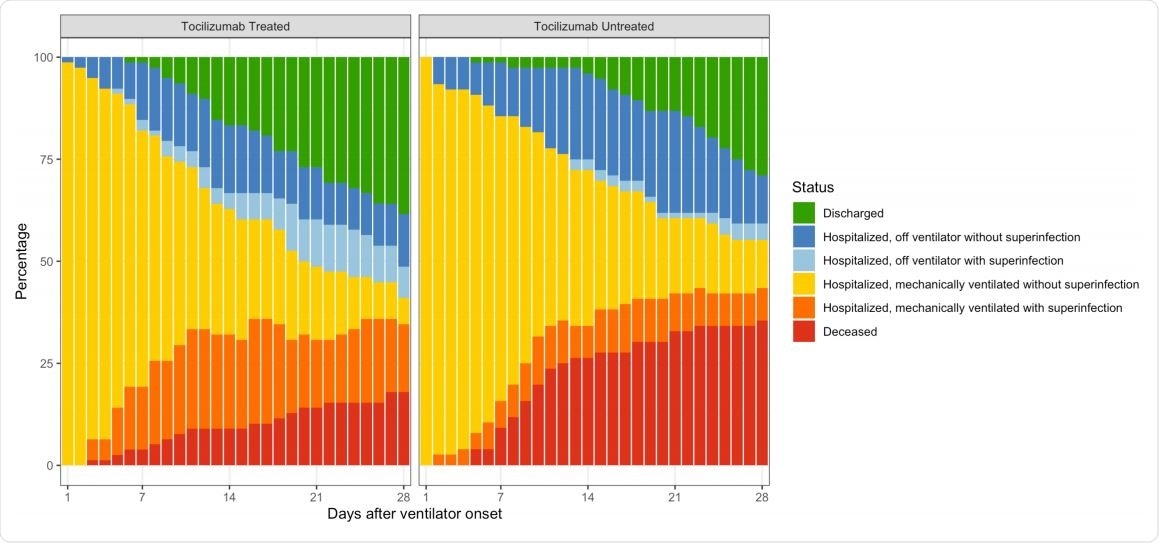
The distribution of patient status, by number of days after onset of mechanical ventilation through day 28 of follow-up.
Implications and Future Directions
In the current study, therefore, the use of tocilizumab was linked to a higher incidence of superinfection but a reduced likelihood of death. The chances of superinfection are significantly greater with this drug, which emphasizes the need for well-powered clinical trials before the drug can be accepted as safe and effective in the treatment of this condition in critically ill patients.
This is the first controlled analysis directly comparing the safety and efficacy of tocilizumab for mechanically ventilated COVID-19 patients. However, the impact of early administration of tocilizumab on the requirement for mechanical ventilation, the most appropriate dose, and regimen, are questions that still beg an answer. This trial should encourage further urgent research into the role of tocilizumab and may influence the current use of this drug, pending such results.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Somers, E. C. et al. (2020). Tocilizumab For Treatment Of Mechanically Ventilated Patients With COVID-19. medRxiv preprint. doi: https://doi.org/10.1101/2020.05.29.20117358. https://www.medrxiv.org/content/10.1101/2020.05.29.20117358v1
- Peer reviewed and published scientific report.
Somers, Emily C, Gregory A Eschenauer, Jonathan P Troost, Jonathan L Golob, Tejal N Gandhi, Lu Wang, Nina Zhou, et al. 2020. “Tocilizumab for Treatment of Mechanically Ventilated Patients with COVID-19.” Clinical Infectious Diseases, July. https://doi.org/10.1093/cid/ciaa954. https://academic.oup.com/cid/article/73/2/e445/5870306