Antibodies can be seen as an outstanding solution that nature has found to the problem of molecular recognition. As such, they have a phenomenal ability to bind to their target molecules.
We have therefore set out to harness their power to recognize amyloid beta oligomers, which are elusive particles closely associated with Alzheimer’s disease.
Image Credit: Atthapon Raksthaput/Shutterstock.com
What happens in Alzheimer’s disease?
The cognitive and functional impairment caused by Alzheimer’s disease can be traced back to a progressive neuronal damage in the patients.
The origins of this damage are still debated, but many think that they are related to the aberrant aggregation of proteins in the brains of the affected individuals.
What are amyloid-beta oligomers?
Among the proteins that aggregate in Alzheimer’s disease, a very special place is occupied by a particular protein fragment known as amyloid-beta.
Amyloid-beta accumulates in the characteristic plaques found in the brains of Alzheimer's patients. Although the presence of these plaques is a hallmark of the disease, the greater toxicity to neurons and other brain cells comes from much smaller assemblies of amyloid-beta, known as amyloid oligomers.
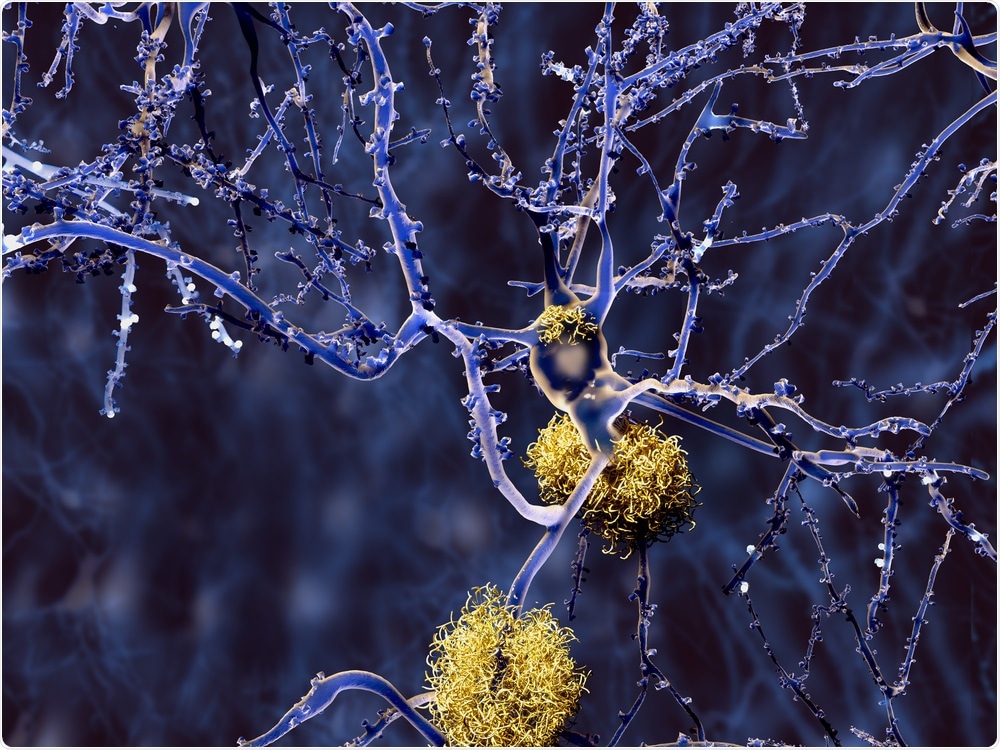
Image Credit: Juan Gaertner/Shutterstock.com
There is currently no treatment to prevent, stop, or slow Alzheimer’s. Why has making progress in Alzheimer’s disease been so difficult?
Alzheimer’s is an age-related disease that can be associated with the progressive impairment of the complex quality control system in our bodies that maintains proteins in their functional states.
Because this system is made up of hundreds, if not thousands, of different components, it has been very challenging to single out actionable targets for therapeutic intervention.
What is the amyloid hypothesis and why has it not been validated?
According to the ‘amyloid hypothesis’, which has been prevalent in the field for the last three decades, the process of aggregation creates small clumps of amyloid-beta that initiate a series of pathological events that eventually result in the death of neurons.
A central role in the amyloid hypothesis is played by amyloid-beta oligomers, which are highly elusive particles that are extremely difficult to observe. Without knowing for certain the positions and numbers of the oligomers it has been highly challenging to prove or disprove the amyloid hypothesis.
One notable example is the failure of clinical trials of drugs targeting amyloid-beta. Without knowing whether or not these drugs can specifically reduce the number of oligomers in treated patients, it is not possible to draw conclusions on the validity of the amyloid hypothesis.
Why is it so important to develop quantitative methods for recognizing oligomers?
It is quite possible, and indeed the central aspect of the amyloid hypothesis, for amyloid-beta oligomers to be the main pathogens in Alzheimer’s disease.
In any case, they may serve as biomarkers by reporting on the progression of the disease from patient samples. By being able to detect and quantify them we will have a much greater control on both the disease state and on the efficacy of the treatments against it.
What is the antibody you have designed and how did you design it?
The antibody that we have designed is capable of binding specifically to amyloid-beta oligomers, thus opening the possibility of detecting their presence and of quantifying their numbers.
Since these oligomers are transient and highly heterogeneous, it is very challenging to use traditional discovery methods to generate antibodies capable of binding them.
We have thus developed a computational approach that has enabled us to design rationally an antibody that binds to a very special region of amyloid-beta that is accessible in the oligomers but not in the larger assemblies of this protein fragment.
.jpg)
Image Credit: Juan Gaertner/Shutterstock.com
How does this antibody recognize Alzheimer’s disease?
The antibody can be used to diagnose Alzheimer’s disease by detecting the presence of an aberrant number of oligomers.
What enables the antibody to quantify oligomers in both in vitro and in vivo samples?
This quantification is possible because the antibody, in essence, binds specifically to the oligomers even in complex samples. Therefore, there is a correlation between binding events and oligomer numbers.
How could this tool be used to discover more drugs for Alzheimer’s treatment and improve clinical trials?
There are at least three major ways.
The first is to aid the process of drug discovery, as by measuring whether a drug candidate can reduce the number of oligomers in preclinical models, a mechanism of action consistent with the amyloid hypothesis can be demonstrated.
The second is in the selection of patients for clinical trials in the presymptomatic phase, before the manifestation of cognitive impairment. In this sense, oligomers can be used as biomarkers.
The third is that one can monitor the efficacy of a drug in reducing the number of oligomers in the treated patients with respect to the placebo group, and to correlate this reduction to the primary cognitive and functional endpoints.
Image Credit: Sisacorn/Shutterstock.com
Do you see a brighter future for making progress in Alzheimer’s research and how will your research influence any progress?
I am optimistic about the amyloid hypothesis, and about the possibility that this new antibody could help validate it by enabling both drug discovery programs aimed at reducing amyloid-beta oligomers.
What are the next steps for your research?
We consider this antibody as the first in a series of increasingly potent ones, which we are now working to develop.
Where can readers find more information?
These web pages provide information about our research:
https://www.ch.cam.ac.uk/chemistry-of-health
https://www.cmd.ch.cam.ac.uk/
http://www-vendruscolo.ch.cam.ac.uk/
About Professor Michele Vendruscolo
I am a Professor of Biophysics at the University of Cambridge, where I moved nearly 20 years ago.
I am Director of the newly established Chemistry of Health Laboratory, whose mission is to translate into clinical applications the research in chemical biology carried out in the University, and Co-Director, together with Prof. Tuomas Knowles, of the Centre for Misfolding Diseases, which I founded with him and with the late Prof. Sir Christopher Dobson.
Our work is aimed at establishing the fundamental principles of protein aggregation, and at exploiting these principles to develop methods for drug discovery in neurodegenerative diseases.