COVID-19 disease has lower case fatality rates when compared to SARS and MERS, which are other serious diseases caused by coronaviruses. Yet COVID-19 is much more infectious, as SARS-CoV-2 spreads much more easily among humans and leads to higher case numbers overall.
Passive immunization with the use of convalescent plasma containing neutralizing antibodies – obtained from individuals that have successfully recovered from the infection – was used extensively during SARS and MERS outbreaks; therefore, it is of no wonder that this approach was immediately proposed as a possibility in treating COVID-19.
The effectiveness of such convalescent plasma therapy may be the best when administered early during the infection process. On the other hand, passive immunization may actually cause harm to the patient when the plasma used contains low antibody titers, and the infusion is administered late during the disease progression.
This is why researchers from the Karolinska Institutet in Sweden and the Institute for Health & Welfare in Finland decided to review all available studies using convalescent plasma transfusion in the treatment of COVID-19, and also to estimate the association between age and disease recovery time following plasma transfusion.
Systematic literature appraisal
A systematic literature review was conducted with the use of the COVID-19 related open-resource literature database LitCovid (curated by the National Center for Biotechnology Information) to pinpoint studies using convalescent plasma as a treatment for COVID-19 patients.
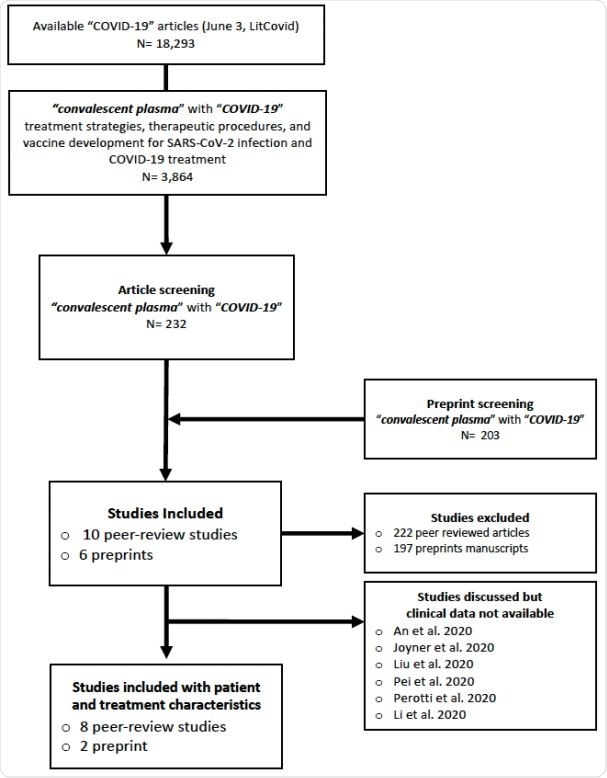
Flow chart of the systematic review study selection. .
The researchers managed to retrieve and classify all COVID-19 related patient and treatment specificities from previously reported studies. Albeit there were 18,293 identified COVID-19-related papers, only ten studies on a total of 61 patients (describing the results of the convalescent treatment for COVID-19) warranted the inclusion in this systematic review.
A statistical Poisson model was developed in order to evaluate the potential association between the patient age (as it is known that the older age is the most common risk factor for COVID-19 mortality) and recovery time after the introduction of convalescent plasma treatment by using data extracted from the literature.
Fewer symptoms and better viral clearance
The primary study finding is that convalescent plasma treatment was consistently safe and resulted in an immediate recovery response in basically half of all the patients included in this review. Shorter time to recovery was significantly associated with patients under the age of sixty.
"We found that patients over the age of sixty who received convalescent plasma treatment for COVID-19 had a significantly prolonged recovery estimated by viral clearance (from 10 to 29 days since the first dose of convalescent plasma) compared to younger patients, who recovered from the infection in less than a week after receiving treatment", emphasize study authors.
Critical observations were a decrease in severe COVID-19 symptoms and clearance of SARS-CoV-2 genetic material. No severe adverse effects after using convalescent plasma were observed, although concomitant treatments were reported in all studies that were included in this report.
In addition, existing comorbidities and patient's gender seemed to exert limited effects on recovery time. Also, there was no association between the recovery time and receiving convalescent plasma either early or late since the onset of COVID-19.
Implications and the need for further research
Although a limited amount of data hampered a comprehensive appraisal of safety and feasibility for the use of convalescent plasma in the treatment of COVID-19, the results in this rather comprehensive review may be considered favorable and significant; hence, this type of therapy can be endorsed.
Nonetheless, a paramount concern is that COVID-19 clinical trials are at the moment implemented primarily due to urgent treatment needs, at the expense of rigorous scientific, ethical, and evidence-based standards. Consequently, this can lead to an increased risk of (hitherto unknown) adverse events, viral transmission, or even complemented-mediated tissue damage.
"Another important caveat related to plasma transfusion treatment for COVID-19 patients, which warrants future evaluation, is the possible transfusion-associated circulatory overload, a common serious adverse effect of transfusion", caution study authors in their medRxiv paper.
In any case, further randomized clinical trial data that follows rigorous ethical standards are urgently needed, and future research endeavors should include a more significant number of patients (especially those in the early phases of the infection) that do not receive multiple overlapping therapies.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.