The potential role the signature might play in COVID-19 pathology and progression might also support therapeutic strategies currently being trialed at St Thomas’ Hospital, say Adrian Hayday and colleagues.
The findings can be accessed in a pre-print version of the paper available on medRxiv*, while the article undergoes peer review.
The COVID-19 pandemic
Human-to-human transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – the causative agent of COVID-19 – has triggered emergency responses globally as the virus continues to infect millions of people worldwide.
Although most infected individuals recover after only experiencing mild or even no symptoms, vast numbers have developed severe complications, including respiratory failure and immune system regulations that have caused death in hundreds of thousands of cases.
The limitation of such outcomes is achieved through the development of antivirals, vaccines, and immunomodulating drugs. An essential part of developing such approaches is understanding the host-pathogen relationship, a core component of which is the immune response.
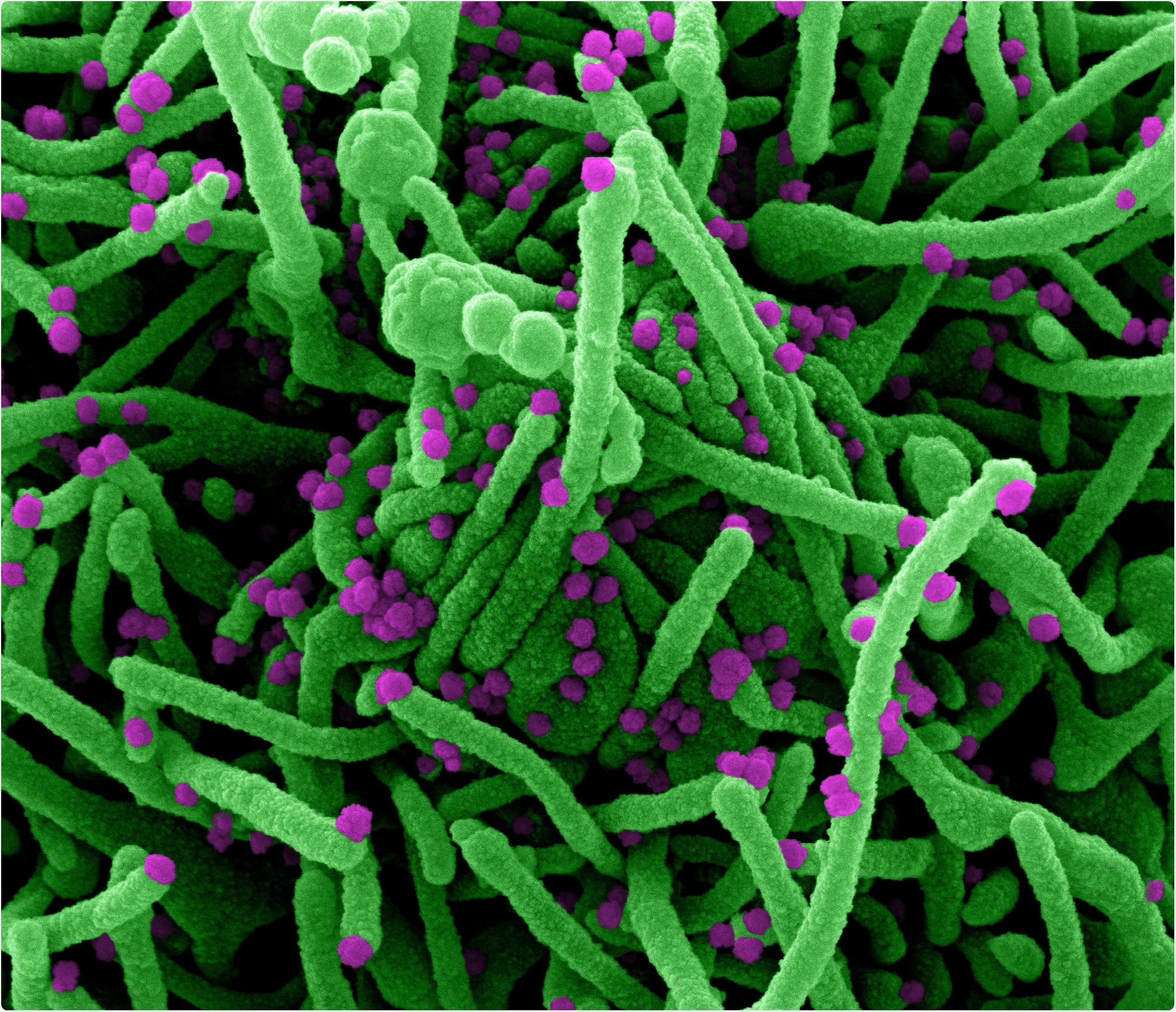
Novel Coronavirus SARS-CoV-2 Colorized scanning electron micrograph of a cell (green) infected with SARS-COV-2 virus particles (purple), isolated from a patient sample. Image captured at the NIAID Integrated Research Facility (IRF) in Fort Detrick, Maryland. Credit: NIAID

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Recovery from SARS-CoV-2
In the case of SARS-CoV-2, a full recovery probably reflects the involvement of protective B cell and T cell responses. Indeed, several patients who became ill with SARS-CoV-1 in 2002-2003 were shown years later to have SARS-CoV-1-specific antibodies and T cells.
In this context, some hospitalized COVID-19 patients suspected to be deficient in virus-neutralizing antibodies have received infusions of antibody-rich plasma from patients who recovered from the disease.
Previous studies have reported immune responses and immune deficits, including T cell depletion, among patients infected with SARS-CoV-2, particularly individuals who have required hospitalization.
The team’s hypothesis
These findings, combined with knowledge about SARS-CoV-1, led Hayday and colleagues to hypothesize that despite heterogeneity in factors such as gender, age, comorbidity, and COVID-19 disease stage, patients infected with SARS-CoV-2 might share a core immune signature that could provide specific insights into the host-pathogen relationship.
To find the signature, the team considered two types of human immune responses, each informed by high-throughput analyses. The first type was the immune-protective responses induced by vaccination or viral infection. The second was sepsis, given that this is an outcome of dysregulated immune responses, including the suppression and depletion of B and T cells.
“Although the clinical presentation of COVID-19 differs from sepsis, we hypothesized that a COVID-19 associated immune signature might share distinct traits with the sepsis immunophenotype,” writes the team. “Identifying those traits might reveal key aspects of the SARS-CoV-2-host relationship, potentially guiding much-needed treatment strategies.”
To test the hypothesis, the researchers analyzed blood samples taken from 63 Covid-19 patients hospitalized at Guy’s and St Thomas’ Trust Hospitals. They compared the results with those of samples taken from 55 healthy, non-hospitalized controls, a small proportion of whom had recovered from COVID-19.
What did the study find?
The team reports that across this highly heterogeneous cohort, a shared core COVID-19 immune signature was identified.
The signature features some elements of previously reported COVID-19 immunophenotypes, combining traits typical of vaccine or viral infection and sepsis with some less frequently cited elements of sepsis. The latter included plasmacytoid dendritic cell depletion, basophil depletion, and hyperactivation of CD4+ and CD8+ T cells, accompanied by selective T cytopenia.
The authors say the blending of immunoprotective and immunopathogenic traits was exemplified by almost universal upregulation of the inflammatory cytokine interferon gamma-induced protein 10 (IP10), as has been observed in cases of SARS-CoV-1 and the Middle East respiratory syndrome.
Furthermore, some specific parameters correlated with the progression of COVID-19, including over-expression of IP10, plasmacytoid dendritic cell depletion, basophil depletion, and T cell proliferation.
By contrast, these components of the immune signature were not observed in healthy controls, including among controls who had recovered from infection. Even those who had been hospitalized for non-COVID-related respiratory infections did not have elevated IP10.
“Thus, an immuno-prognostic Covid-19 test might have high specificity,” writes the team.
The signature could aid prognosis prediction and patient stratification decisions
The researchers say the novel immune signature could provide specific insights into the SARS-CoV-2-host relationship and aid prognostic disease prediction and risk-based patient stratification.
Furthermore, “if the immunological signature not only tracks but contributes COVID-19, our findings support therapeutic strategies to boost T cell competence, e.g., by use of IL7, currently on trial at St Thomas’ Hospital, possibly combined with IP10 antagonism,” concludes the team.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources