The ongoing COVID-19 pandemic has taken an enormous toll in terms of human lives, productivity, and economic disruption. Scientists have been working intensively to find effective and safe therapeutics for the viral infection, mostly by inhibiting the entry of the virus into the host cell. Now, a new study by researchers at Tulane University and published on the preprint server bioRxiv* in June 2020 reports the potential role of an integrin inhibitor ATN-161 to prevent ACE2-S protein binding.
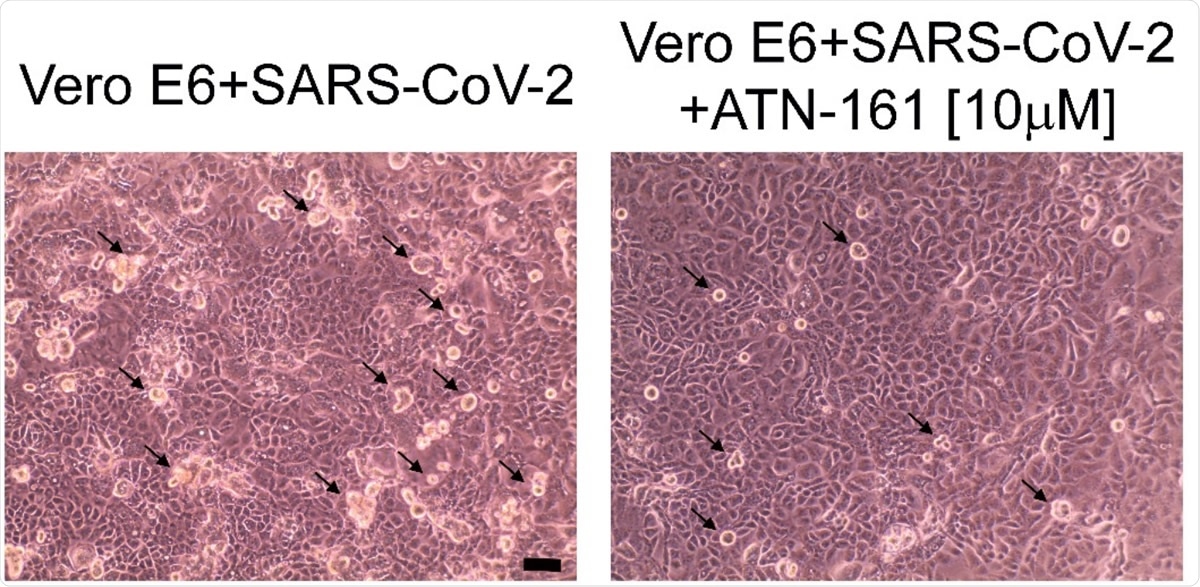
Representative phase-contrast microscope images of VeroE6 cells 24 hours post-infection with and without 10 µM ATN-161 treatment. Black arrows indicate some of the visible viral cytopathic effect (rounded, phase bright cells). Scale bar is 10 µM.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
SARS-CoV-2 Entry into the Cell
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) enters the host cell via the ACE2 receptor, binding to it via the spike protein, which has a receptor-binding domain (RBD). This entry mechanism also involves integrin binding, since the spike protein has an integrin-binding motif that attaches to the RGD (arginylglycylaspartic acid) pocket on the integrin.
The Role of Integrins in SARS-CoV-2 Infection
Integrins are extracellular receptors, found in the extracellular matrix around the cells in every tissue. As such, integrins are present in the respiratory epithelium and vascular endothelium. The β-1 integrin family is also found to function in close relation to the ACE2 molecule.
ATN-161 and ACE2/ α5β1 Binding
The peptide called ATN-161, which is a fibronectin derivative, is known to bind to and block some integrins, such as α5β1. As a result, it has been used to examine the process of viral replication.
ATN-161 does not bind to the RGD site, being a non-competitive α5β1 inhibitor. The ACE2 receptor also binds non-competitively to α5β1, and not via RGD. The researchers wanted to find out if the entry of the virus is promoted by the binding of the viral spike protein to the α5β1 integrin, which is, in turn, bound to the ACE2 molecule.
This would bring the virus in proximity to the ACE2 protein. If so, the presence of ATN-161 will block SARS-CoV-2 infection by preventing integrin-binding with the spike protein, and the preliminary ACE2- α5β1 association as well.
ATN-161 Inhibits Spike/α5β1 Binding
The study used ELISA to quantify the spike protein-ACE2 and spike- α5β1 binding. First, they incubated the α5β1 integrin with both trimeric spike protein and ATN-161. The spike protein and fibronectin have almost the same affinity for the α5β1 integrin, even though fibronectin is the native ligand of this integrin. ATN-161 affects the binding of the α5β1 to the spike protein in a dose-dependent manner, the maximum inhibition being seen at 100 nM.
ATN-161 Inhibits ACE2/ α5β1 Binding
Next, they looked at the binding of ACE2 to α5β1, using a mix of ATN-161 at varying concentrations, and human ACE2 (hACE2) added to plates coated with α5β1. They found that ATN-161 inhibits the binding of ACE2 to α5β1, with increasing inhibition as the dose of ATN-161 increases. The trimeric spike protein-ACE2 binding was unaffected by the presence of ATN-161, whereas the binding of the monomeric form was inhibited.
ATN-161 Mitigates Severity of Infection
When a cell culture was exposed to SARS-CoV-2 in the presence of ATN-161, they found the latter reduced effective viral loads following infection, with an IC50 (half-maximal inhibitory concentration) of 3.16 µM, and the half-maximal effective concentration being comparable to that of the approved drug remdesivir.
Finally, they observed that when first exposed to ATN-161, infected cells remained more viable. Even at a low concentration of 1 µM, ATN was associated with a low copy number. When 10 µM of ATN-161 was used, the cytopathic effect was reduced, resulting in a lower number of rounded bright cells on phase-contrast microscopy.
Implications and Future Directions
This is the first time that the SARS-CoV-2 has been seen to interact with integrins. The study shows that the spike protein binds to both the α5β1 as well as to the α5β1-hACE2 complex. The inhibitor ATN-161 prevents this binding effectively, and in vitro experiments show that it prevents cell infection by SARS-CoV-2 as well.
When a cell culture is exposed to ATN-161 before being infected with this virus, the infected cells retain greater viability, with higher ATP production, which is a sign of cell health. The cytopathic effects produced by the virus are also less intense in such cells.
Overall, therefore, the findings indicate that this molecule should be tested in vivo for its efficacy in treating COVID-19, especially since it has already been shown to be effective therapeutically against the porcine hemagglutinating encephalomyelitis virus, which is a betacoronavirus closely related to SARS-CoV-2, and has successfully been used in human clinical trials for cancer therapy.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Beddingfield, B. et al. (2020). The Integrin Binding Peptide, ATN-161, as a Novel Therapy for SARS-CoV-2 Infection. bioRxiv preprint. doi: https://doi.org/10.1101/2020.06.15.153387. https://www.biorxiv.org/content/10.1101/2020.06.15.153387v1
- Peer reviewed and published scientific report.
Beddingfield, Brandon J., Naoki Iwanaga, Prem P. Chapagain, Wenshu Zheng, Chad J. Roy, Tony Y. Hu, Jay K. Kolls, and Gregory J. Bix. 2021. “The Integrin Binding Peptide, ATN-161, as a Novel Therapy for SARS-CoV-2 Infection.” JACC: Basic to Translational Science 6 (1): 1–8. https://doi.org/10.1016/j.jacbts.2020.10.003. https://www.sciencedirect.com/science/article/pii/S2452302X2030440X