Researchers in China have demonstrated the ability of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to interact with the host cell receptors of multiple species of animals.
The authors say it is this feature of the virus that significantly complicates efforts to pinpoint both the original and intermediate hosts so that their potentially diverse cross-species transmissibility can be evaluated.
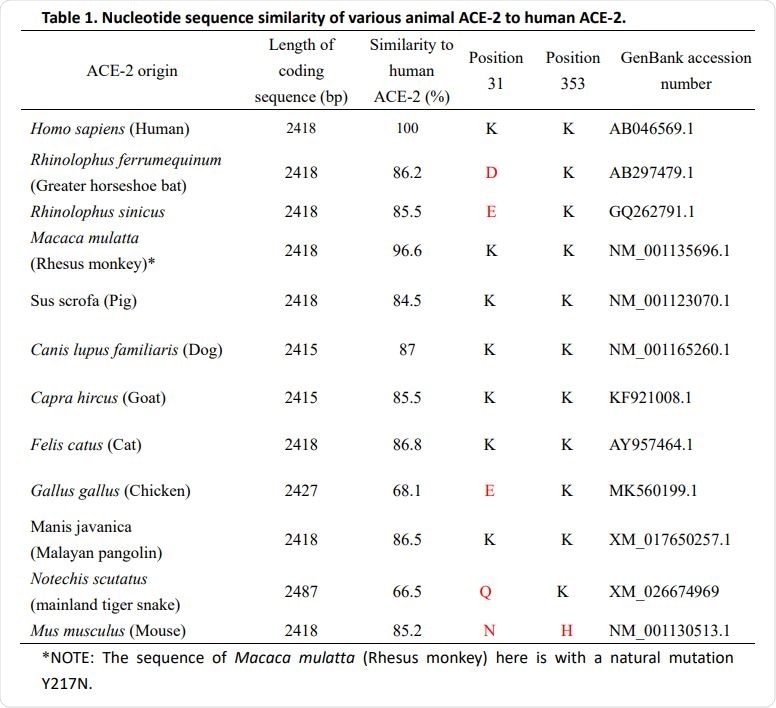
The team’s study of eleven different animal species found that the SARS-CoV-2 host cell receptor – angiotensin-converting enzyme 2 (ACE2) – mediated viral entry in the Chinese horseshoe bat, domestic cat, dog, pig, goat, and Malayan pangolin.

A Pangolin. Image Credit: 2630ben / Shutterstock

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The researchers from the Chinese Academy of Agricultural Sciences, the Chinese Center for Disease Control and Prevention, and various Chinese universities say this is the first time ACE2-mediated SARS-CoV-2 entry has been demonstrated in the pangolin, a finding that supports previous studies implicating this animal as the original host.
Species with ACE2 that did not enable viral entry included the greater horseshoe bat, chicken, mainland tiger snake, and house mouse.
The team also found that a naturally mutated version of the ACE2 receptor in the Rhesus monkey conferred resistance to infection, indicating the importance of studying this ACE2 variation in the future.
A pre-print version of the paper is available in bioRxiv*, while the article undergoes peer review.
Identifying potential hosts "will be scientifically very important"
Since COVID-19 first emerged in Wuhan, China Late last year, the pandemic has infected more than 8.28 million people and caused more than 446,000 deaths.
While these numbers are only expected to increase as the pandemic continues to sweep the globe, researchers have still not identified the precise origin of the virus or the intermediate hosts that might potentiate the cross-species transmission, which would help to inform control and prevention methods in the future.
“Discovering the potential intermediate animal hosts of SARS-CoV-2 and evaluating their possible cross-species transmissibility will be scientifically very important,” say Xuhui Cai (Harbin Veterinary Research Institute of Chinese Academy of Agricultural Sciences) and colleagues.
“Unfortunately, we know little about this. Currently, there are no suitable animal models for SARS-CoV-2 infection.”
Cell susceptibility to infection an important consideration
Bats have been recognized as a potential natural reservoir for the virus, but more recent studies have pointed towards the pangolin as the origin.
When retracing the potential sources of coronaviruses, it is important to consider the cell susceptibility to infection conferred by receptors in suspected animal species.
For example, before clarifying that the Chinese horseshoe bat was the origin of SARS-CoV-1, researchers first assessed the susceptibility to infection conferred by ACE2 in various bat species. This revealed that ACE2 in the Chinese horseshoe bat was responsible for the susceptibility, thereby confirming this species was the original viral reservoir.
For the current study, Cai and colleagues assessed the ability of patient-derived SARs-CoV-2 to infect non-susceptible human cell lines engineered to express ACE2 from twelve different species of animals, including humans.
Susceptibility to SARS-CoV-2 of HEK293T cells conferred by different 2 species of ACE2. HEK293T cells were transfected with plasmids expressing 3 indicated ACE2. Cells were infected with 0.5 MOI of SARS-CoV-2 24 h after the 4 transfection, and were detected for the replication of SARS-CoV-2 by IFA.
What were the main findings?
The study found that ACE2 from the greater horseshoe bat failed to mediate SARS-CoV-2 entry, whereas ACE2 from the Chinese horseshoe bat did mediate entry.
This “demonstrated that not all species of bat were sensitive to SARS-CoV-2 infection,” said Cai and colleagues.
The ACE2 receptor derived from the Malayan pangolin also mediated SARS-CoV-2 entry.
Given that the SARS-CoV-1 engages the ACE2 receptor in both humans and the Chinese horseshoe bat, “the ability of pangolin ACE2 to confer the susceptibility to SARS-CoV-2 entry increases the possibility that SARS-CoV-2 originated from pangolins,” writes the team.
What about the Rhesus monkey?
A recent study found that a natural mutation (Y217N) in the ACE2 receptor of the Rhesus monkey down-regulated the expression of the receptor and significantly reduced cellular susceptibility to SARS-CoV-2 infection.
However, in this study, expression levels of this mutated ACE2 from Rhesus monkeys was similar to the expression level in cells transfected with human ACE2.
“Therefore, the failure of monkey ACE2 isoform to convert the cell susceptibility to SARS-CoV-2 entry is not due to the poor expression of the receptor as previously speculated,” write the researchers, who say the mechanism requires further investigation.
What are the study implications?
The researchers say their study shows that SARS-CoV-2 was able to engage ACE2 receptors across a broad range of animal species, highlighting the challenges faced in identifying original and intermediate hosts to help inform future control and prevention.
“Of note, here is the first report that ACE2 of Pangolin could mediate SARS-CoV-2 entry, which increases the possibility that SARS-CoV-2 may have a pangolin origin,” says the team. “It is also important to detect the expression ratio of the Y217N ACE2 to the prototype in Rhesus monkeys to be recruited for studies on SARS-CoV-2 infection.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources