A new study by researchers in the Netherlands and published on the preprint server bioRxiv* in June 2020 describes the differences in T and B cell responses seen in patients with severe COVID-19.
The current pandemic of COVID-19 caused by the RNA virus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has by now, in just six months, caused more than 8.77 million cases and over 464,000 deaths. With its rapid transmission and high mortality in some segments of the population, COVID-19 has become the first worldwide scourge of the current century.
Viral Binding and Replication
The virus binds to human cells via the ACE2 receptor and the protease TMPRSS2. Following successful infection, the virus undergoes active replication, and the infectious particles are shed, to infect more cells.
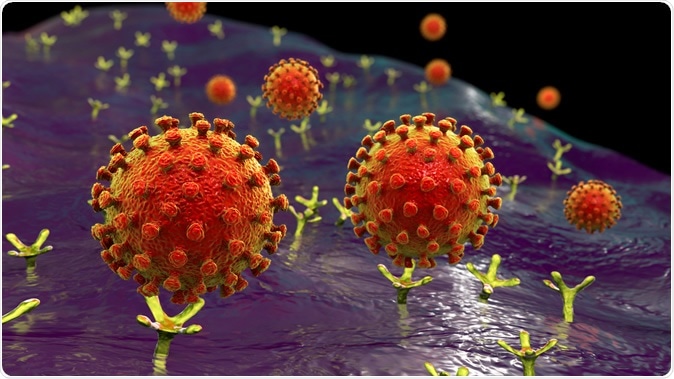
SARS-CoV-2 viruses binding to ACE-2 receptors on a human cell, the initial stage of COVID-19 infection, conceptual 3D illustration credit: Kateryna Kon / Shutterstock
During the process of viral spread within the body, damaged virus-containing cells produce specific patterns of molecules, called damage-associated molecular patterns (DAMPs). These trigger immune and inflammatory cascades, which in turn lead to the secretion of cytokines and cell chemicals that recruit more immune cells to the site of viral replication and injury.
As these cells enter the act, they promote the inflammatory response, and in severe COVID-19, there is apparently a dysregulated or hyperinflammatory reaction. This results in a cytokine storm, with the massive levels of cytotoxic chemicals exerting noxious effects on multiple tissues and organs, leading to acute respiratory distress syndrome (ARDS), dysfunction of multiple organs, and even death.
T Cells During COVID-19
During this process, T cells are attracted to the lungs, especially those that are specific for their antiviral cytotoxic effects. These are first primed by the dendritic cells that present viral antigens in the lymph nodes of the lung, training them to recognize viruses, and then to kill the host cells within which the viruses are replicating. This will end the spread of the virus.
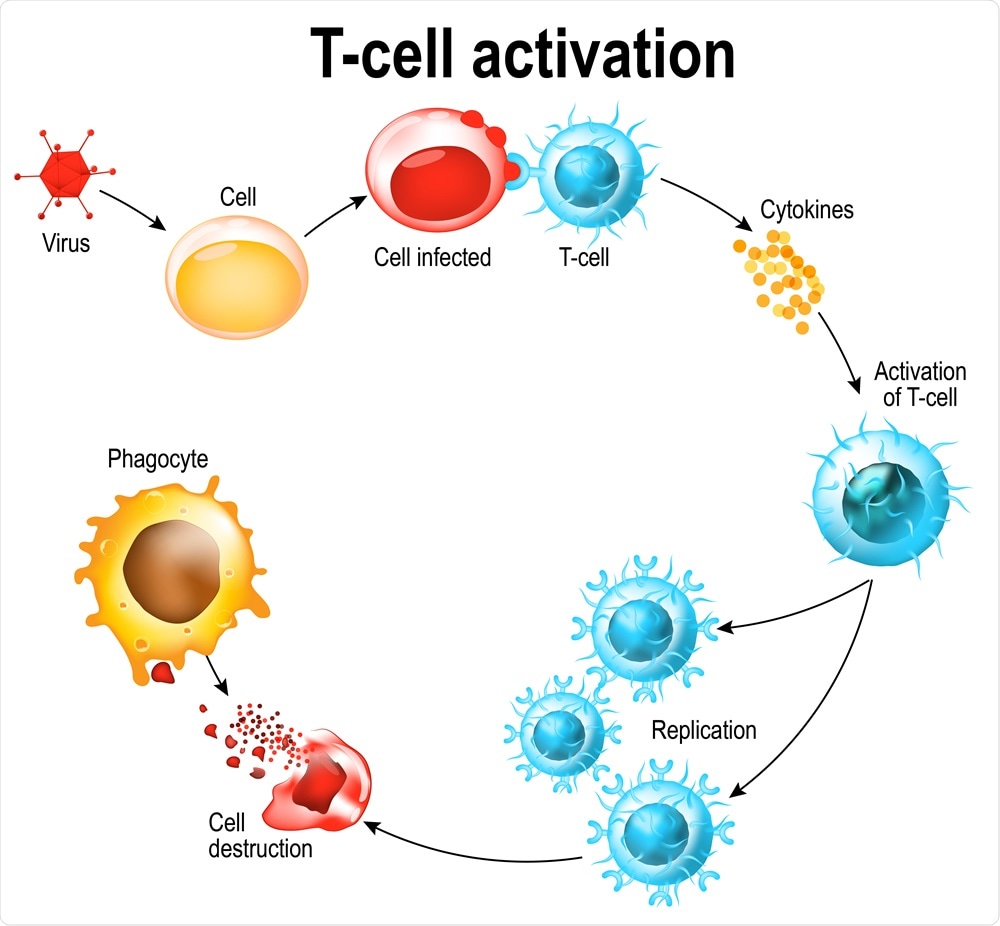
Activation of T-cell leukocytes. T-cell encounters its cognate antigen on the surface of an infected cell. T cells direct and regulate immune responses and attack infected or cancerous cells. Image Credit: Designua / Shutterstock
Laboratory tests have found that both CD4 and CD8 T cells, as well as activated T cells, are present in the blood of COVID-19 patients at 1-2 weeks from the start of symptoms. These cells produce mainly Th1 cytokines. CD8 cells are directly cytotoxic, while CD4 cells, especially Tfh cells, have the additional function of recruiting and class-switching B cells. The cells increase in the blood of infected patients over time, with a raised level in critical COVID-19 patients compared to healthy people.
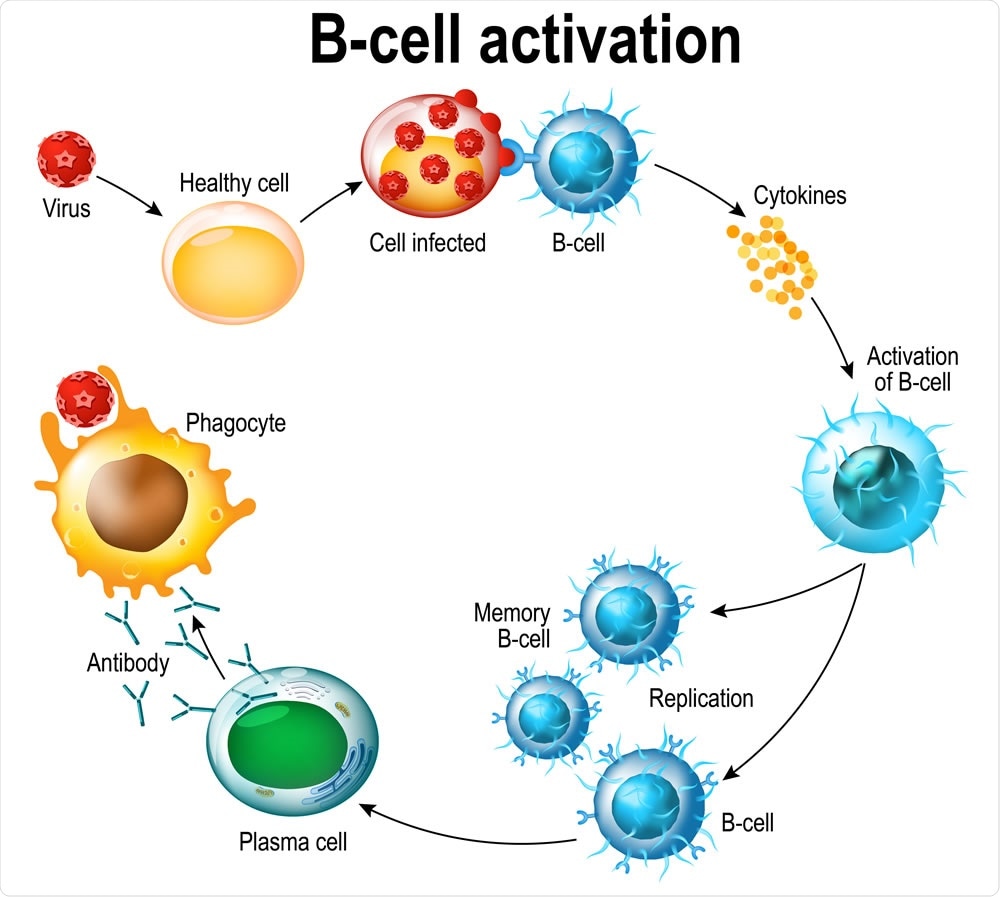
Activation of B-cell leukocytes: lymphoblast, activation, memory B-leukocyte, virus, plasma cell, antibody, antigen, and naive lymphocyte. Image Credit: Designua / Shutterstock
Lymphopenia, or reduced lymphocyte count, is a common feature in many severe COVID-19 patients, which may be the result of the migration of enormous numbers of T cells to the lungs and other sites of inflammation. Alternatively, it could be due to the death of activated T cells on a large scale within the secondary lymphoid organs such as the spleen. Blood samples from these patients show a high build-up of exhausted T cells with a subnormal range of functions, which lends support to the idea that antigen-specific T cells are vital to the immune control of this infection.
The Current Study
The current study was aimed at understanding the correlation between the T cell responses in COVID-19 with the type of symptoms, so as to define better how these cells help clear the virus and produce symptoms of immune-related organ damage.
The researchers looked at 56 samples from COVID-19 patients, with 21 recovered mild cases, 14 recovered after severe hospitalization-requiring cases, and 21 critically ill requiring intensive care unit (ICU) admission.
CD4 cells are increased in mild, moderate, and ICU patients, but with a trend towards increased IL-4 and IL-21 in the last group, perhaps because of aging effects.
The researchers say, “We observe major differences in the quality and quantity of these responses across the different clinical groups.”
The antibody titers were significantly linked to CD4 T cells specific to the Spike (S) protein antigen, and these patients typically had mild symptoms.
However, the balance between T and B cell responses was lost in critically ill COVID-19 patients, with low CD4 and CD8 counts and reduced levels of cell signaling molecules such as IFN-γ, IL4, and IL-21. This reflects both a positive effect on immune cell recruitment and a negative effect on viral replication.
It can also reduce the expression of ACE2 on the host cell, as also IL-4. However, IL-21 can act on B cells and affect the responses at the germinal center. The inhibitory marker PD1 expression is also enhanced, most noticeably in ICU patients and least in recovered mild infections. All of this supports the conclusion that the T cell response is weakened in critically ill COVID-19 patients.
T Cells in Bronchoalveolar Lavage Fluid
Cross-reacting T cell responses are also present in pre-pandemic samples. The bronchoalveolar lavage fluid (BALF) of these patients shows a large number of naïve T cells, unlike in normal conditions, and few memory T cells. This is associated with reduced vascular integrity and breakdown of the epithelial barrier, with alveolar leakage.
The CD4 cells explicitly targeting the S antigen in both BALF and peripheral blood mononuclear cells (PBMC) also showed, like the above, a low frequency of resident memory T cells, again indicating the presence of leaking blood vessels. In other words, the T cells in BALF come from the circulating pool and are not tissue-resident memory cells that offer prolonged protection against the virus.
The findings agree with earlier research showing that T cells were expanded in the BALF of moderate COVID-19 but not critical illness, which could suggest that the low lymphocyte count seen in critical patients is because this expansion is affected, or because certain clones are depleted. Also, more lymphocytes are recruited to the lungs.
Balance and Timing of B and T Cell Activity Essential
The study suggests that both B and T cells must be involved to clear SARS-CoV-2 from the body, and also that T cells are unable to deal with the rapid rise in viral titer in this infection. The peak viral load is from the tenth day from the earliest symptom, unlike earlier coronaviruses, which peaked earlier.
The higher the viral load, the more severe the disease was and the lower the T cell count, and the older the patient. The researchers also observed that patients with severe COVID-19 but who did not need intensive care had a higher specific T cell response against the virus, along with high specific antibody responses, both IgM and IgG. This was in contrast to critically ill patients who had an early and strong antibody response, but a poor T cell response.
In other words, say the researchers, “Timing appears to be crucial as the ratio between viral loads and antibody titers during the early phase of disease may be predictive for disease severity.”
With an inadequate T cell viral clearance and a strong antibody response, coupled with poor innate immunity, as is common in elderly people, the antibodies could end up harming the body without clearing the virus. This difference in T cell counts directed against the S and N viral proteins could be why severe, and critical disease end up differently.
Why Age Confers Increased Risk
The now established risk posed by age is also explained by a reduced T cell response, since the senescence of cross-reactive T cells, the lower production of T cells in response to new viral antigens, and the suppression of T cell responses by the failure to expand or because they enter apoptosis as a result of the cytokine storm, which is common in critically sick COVID-19 patients.
Future Research
Another avenue of interest is the potential role that failed T cell responses could play in the uncontrolled inflammation and immune-mediated organ damage characteristic of critical COVID-19. Circulating Tfh could be crucial for specific antibody production, and their frequencies are highest in ICU patients.
Conversely, B cells may also show an altered response in critical COVID-19, where afucosylated anti-S IgG is produced that promotes antibody-dependent cellular cytotoxicity (ADCC) and show increased binding to Fc receptors. Since these receptors are needed for T cell priming, this might account for the lower count of antigen-specific T cells in these patients. The researchers also suggest that the expression of sugar residues on B cells as a result of Tfh or other T cell-mediated signals, which needs further study.
A better understanding of T and B cells responses, both functional and quantitative, with respect to the timeline of antigenic stimulation, will be essential to assess the usefulness of future vaccines and the disease process in COVID-19.
Important Notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.