Mesenchymal stem cells labeled with fluorescence molecules. Image Credit: Vshivkova / Shutterstock
Scientists worldwide are working at an unprecedented pace to find a vaccine against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of the COVID-19 pandemic. Although several vaccine candidates turned into real frontrunners, there is still a long way until we have one ready for public use.
Such global effort was facilitated by detailed molecular characterization of SARS-CoV-2 since the start of the pandemic. Today we know that, among some other viral constituents, structural nucleocapsid protein (N-protein) can prompt a dominant and long-lasting immune response.
However, major weak spots of current vaccine candidates are low efficiency, immune adaptability, tolerability, and safety. Furthermore, most of the vaccines fail to address the modification that arises in the host during viral replication. All of this means that the preclinical hunt for promising vaccine candidates is far from over.
Recently, Junhua Liu, Huping Jiao, and Xiushan Yin – three Chinese researchers with multiple affiliations – elegantly demonstrated that the modified mesenchymal stem cells expressing SARS-CoV-2 proteins could be used as a novel and effective vaccine platform.
Transfecting mesenchymal stem cells with N-protein
For the purposes of this study, the researchers used allogeneic mesenchymal stem cells that are basically a norm in transplantation-based cell therapy. More specifically, mesenchymal stem cells derived from the human umbilical cord were isolated and evaluated by flow cytometric analysis and functional assay.
After passaging them for four times, primary mesenchymal stem cells were transfected with a plasmid expressing N-protein, achieving average transfection efficacy of 20 percent after 48 hours.
The resulting mesenchymal-SARS-CoV-2-N cells were used for mice injection. A total of one million modified mesenchymal stem cells were inoculated in mice either intramuscularly or subcutaneously in order to enable the production of vaccine antigens in vivo.
Blood samples were taken from mice 20 days after vaccination. At the same time, 5-8 wild mice were randomly selected from the same batch before injection in order to use their sera as negative controls. The enzyme-linked immunosorbent assay (ELISA) was used to appraise the ability to produce antibodies against N-protein.
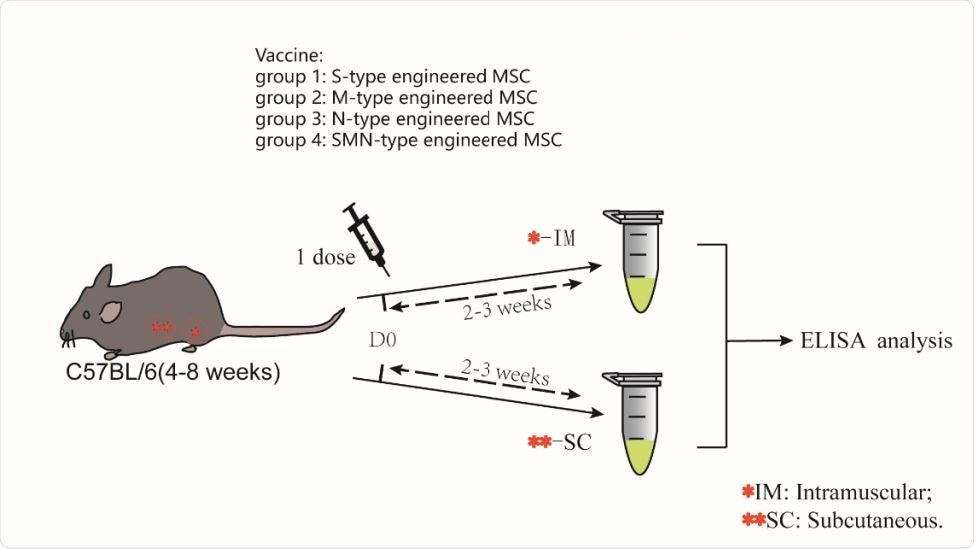
A schematic diagram of the one-dose MSCs injection Four groups of mice were given a single dose of intramuscular injection with several types of engineered MSC. Similarly, the other four groups of mice were given another subcutaneous injection for the same experiment. After two weeks later, blood sample was collected for ELISA.
Robust antibody production against SARS-CoV-2
The results revealed that after 20 days, practically all the immunized mice established antibody production in their serum, and at least half of them showed strong positive antibody expression after one dose of an intramuscular or subcutaneous cell application.
The response after these two types of injection showed similar efficacy. Moreover, mice that were inoculated with an empty vector did not show antibody production against N-protein, and the ELISA signal was comparable to mice that were not injected with anything.
Although a potential risk of any cell-based vaccine is that residual cells present in the body might cause uncontrolled proliferation and malignancy, the study authors also state that the mesenchymal stem cells were cleared by the immune system 20 days after injection. Still, the data to corroborate this claim has not been disclosed in this version of the paper.
A promising strategy for COVID-19 prevention
In any case, the practical research route based on stem cells (as delineated in this report) can enable the presentation of multiple antigen proteins as targets by exploiting the feature of presenting and secreting part of the SARS-CoV-2 antigen protein.
Even more importantly, some of the SARS-CoV-2 proteins mediated and secreted by stem cells may be modified with acetylation and glycosylation processes, mimicking the virus protein modification in vivo. This can, in turn, simulate response much better and increase the success rate of establishing adequate immune protection.
"Combined with the advantages of mesenchymal stem cells in the treatment of diseases, we believe that such stem cells or cell-based vaccines will be a promising strategy to control COVID-19 and other coronaviruses with unknown potential risk, and it is also a promising model for some pandemic diseases", conclude study authors.
It must be noted that after several antibody response tests, these researchers obtained an injection of a set of cocktail-like modified human mesenchymal stem cell line. Consequently, such a strategy truly creates a new paradigm for vaccine design against COVID-19.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.