A new study published on the preprint server medRxiv* in June 2020 shows that variants in the ACE2 gene which encodes the enzyme receptor for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19 disease, are linked to the risk of severe disease.
The ongoing COVID-19 pandemic is still not wholly understood concerning how it is spread, who is at risk, and what determines the severity of the disease. However, it is well established that underlying disease, including type 2 diabetes, cardiovascular disease, and obesity, is associated with more severe illness and a higher risk of death.
ACE2 Receptor and Viral Entry
One common and plausible explanation is that such patients have a higher concentration of ACE2 molecules in their tissues. Since these are the receptors for the virus, essential for its attachment to and entry into the host cell, the importance of these molecules in risk stratification is obvious.
ACE2 receptors bind the spike (S) protein of the virus and are found at high levels in the tissues of the heart and the vascular endothelium, which could be one way by which the virus causes direct injury. The need for virus-ACE2 interaction means that genetic changes that alter the expression of this receptor or the strength with which the virus can interact with the receptor can influence the severity of the infection.
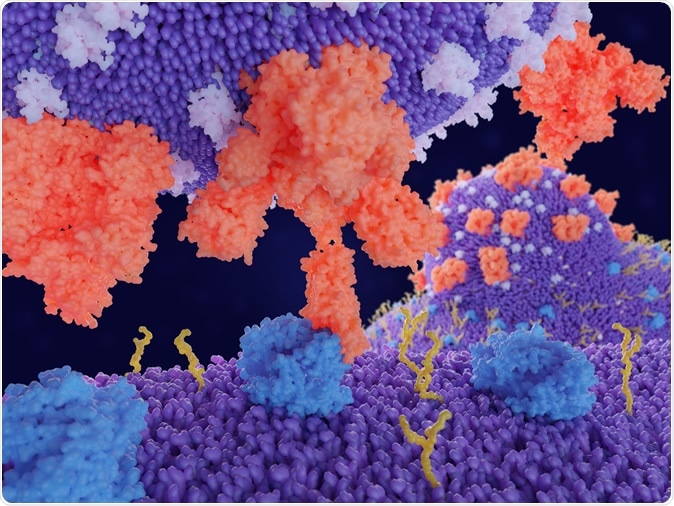
Binding of the coronavirus spike protein(red) to an ACE2 receptor (blue) on a human cell leads to the penetration of the virus in the cell, as depicted in the background. 3d rendering. Image Credit: PDB 3sci / Shutterstock

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
ACE2 is on the X chromosome but is not inactivated so that females have two alleles, and males have one. Earlier studies show that there are different coding variants of ACE2 in different populations where COVID-19 has become endemic. The current study aimed to understand how the functional genetic variation in the ACE2 receptor influences the clinical outcome of COVID-19 infection. The specific outcome was hospitalization for COVID-19 in relation to the dosage of the minor ACE2 allele.
The Study: Analyzing Gene Variants vs. Hospitalization
The researchers from Harvard Medical School, Massachusetts General Hospital, and Case Western Reserve University School of Medicine obtained genetic data from the Partners Biobank database on patients subsequently diagnosed with COVID-19 in consecutive order. Among more than 30, 750 patients with data in this bank, 62 were diagnosed with COVID-19 by reverse transcriptase-polymerase chain reaction (RT-PCR). The median age was 62, with 66% of patients being male.
They examined all the single nucleotide polymorphisms (SNPs) that were significantly linked to transcription of ACE2-encoding mRNA and had a minor allele frequency (MAF) of 5% or more. No coding variants were found at this frequency.
Of the total patients, 23 needed to be hospitalized, or 37%. In this group of patients, there were 61 SNPs, of which ten were significant for the receptor in one or more tissue types. Five of these ten were on a less common form of the gene (minor allele) and were linked to a higher expression of ACE2 and a higher rate of hospitalization for COVID-19, after controlling for the effect of sex and age.
Another SNP on the minor allele was found to be associated with a lower expression of tissue ACE2, and to a lower COVID-19 severity that did not require hospitalization.
Thus, six of ten SNPs that are linked to a change in ACE2 expression in peripheral tissues reflect in altered hospitalization rates.
SNPs and ACE2 Expression Linked to Hospitalization Rates
Earlier studies have demonstrated a higher frequency for alleles that contain SNPs linked to higher expression of ACE2 in East Asia. This finding has been challenged, but single-cell sequencing has shown a greater expression of ACE2 in type II lung alveolar cells from Asian males compared to White or Black donors.
The current study takes these potential population-level differences in ACE2 expression a step further by looking for polymorphisms in the ACE2 locus that may affect the severity of the disease. The researchers sum up: “Genetic variants that have been associated with ACE2 mRNA expression levels associated with disease severity.”
The results are preliminary and not peer-reviewed, they need to be validated in other patient cohorts and in other population groups. The investigators call for more research into how these SNPs alter the expression of ACE2 in lung tissue, as well as in the heart and vascular endothelium. This will help to better understand how ACE2 expression affects COVID-19 infection rates and the severity of disease.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.