As of June 25, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – a causative agent of the ongoing COVID-19 pandemic – infected almost 9.6 million people across 188 countries and territories, according to the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU).
The pace of fundamental and clinical research on the virus is unparalleled, but there is still a lot to learn. For example, susceptibility to COVID-19 in patients with immune-mediated disorders and under treatment with biological therapy is presently unknown.
More specifically, it is still unclear whether patients receiving immunosuppressive therapy are more prone to SARS-CoV-2 infection than the general population, and if they get infected, whether these biological therapies can lead to a higher rate of complications (such as secondary bacterial pneumonia or acute respiratory distress syndrome).
By using a systematic approach, the researchers from the Hospital Universitario Fundación Alcorconin, Universidad Rey Juan Carlos University Spain and Health Center La Rivota in Alcorcon in Spain analyzed the incidence of severe COVID-19 (i.e., necessitating hospitalization) by comparing the cohort of patients receiving biological therapies with the general population.
Biological therapy is a type of treatment that uses substances made from living organisms to treat disease. These substances may occur naturally in the body or may be made in the laboratory. Image Credit: Numstocker / Shutterstock

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Digging through the databases
This study relied on prospective and retrospective data collection approaches from Hospital Universitario Fundación Alcorcon with the use of two administrative databases. One of them contained data of all COVID-19 patients between March and April 2020, while the second one was specific for patients at rheumatology, dermatology, and gastroenterology departments currently receiving biologic therapy.
There were a total of 2,182 COVID-19 cases necessitating hospital care, from which 70.21% required hospitalization, and the rest were admitted to the emergency room only. The mean age was 64 years, there were 45% female cases, and a total death count was 265 (or 12%).
Furthermore, the COVID-19 crude incidence rate (adjusted by sex and age) was estimated for those receiving biological therapy and compared to the general population. The Municipal Registry of Alcorcon was used to calculate the incidence rates for the control group (i.e., the general population).
Biological therapy linked to a lower incidence of severe COVID-19
The main finding of this study was a significantly lower incidence of severe COVID-19 manifestations in the cohort of patients with biological therapy when compared to the general population (with a somewhat higher proportion of women in the biologic therapy cohort).
"This finding is reinforced by the fact that the mean age in the cohort with biological therapy is 10 years older than the mean age in the general population, and it is known that age is the most important risk factor for developing a severe form of the disease", further emphasize study authors in their medRxiv paper.
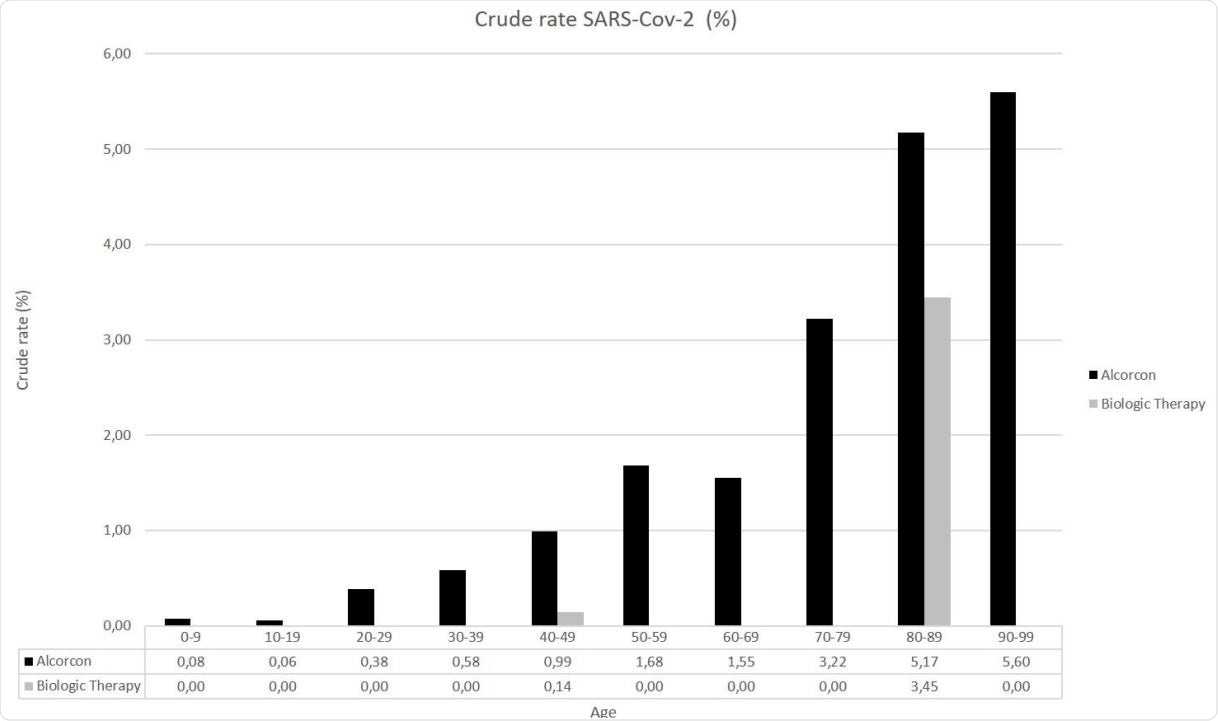
More specifically, the crude incidence rate of COVID-19 cases requiring hospital care in the general population was 1.28%, whereas, in the biological therapy cohort, it was 0.5%. As a result, an incidence rate ratio between these two cohorts was 0.39, which translates to a substantially lower risk of hospitalization with the use of biologics.
Study limitations and implications
Naturally, this study is not without limitations. First of all, only cases from the emergency service and requiring hospitalization have been identified, albeit many rheumatic patients with mild COVID-19 had a self-reported disease, without the confirmation by molecular PCR techniques.
Moreover, a number of COVID-19 cases in the biological therapy cohort were rather low, which hampers the capacity to analyze factors linked with the severe form of the disease. Hence, definite conclusions are still elusive.
Notwithstanding all these issues, the results of this study can be used to support the claim that patients with the immune-mediated disease and receiving biological therapy do not present with the higher risk of severe COVID-19 manifestation, more specifically, that risk is actually lower than for the general population.
But final conclusions await the analysis of a more extensive case series that will incorporate a more significant number of patients on biological therapy. In the meantime, it is essential to bear in mind that this is a rapidly evolving situation where recommendations change as more data become available, which means shared decision-making with patients on biologic therapy is indispensable.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Source:
Journal reference: