As experts in hematology HORIBA UK Ltd, Medical announces that the Company has published a white paper entitled, ‘COVID-19 screening, prognosis and severity assessment with biomarkers for management of patients’. Written in collaboration with academic partners, the paper provides a review of routinely measured biomarkers that can provide additional information to help in the assessment of the severity of COVID-19 infection; therefore, supporting clinical decision making for risk stratification and patient management.
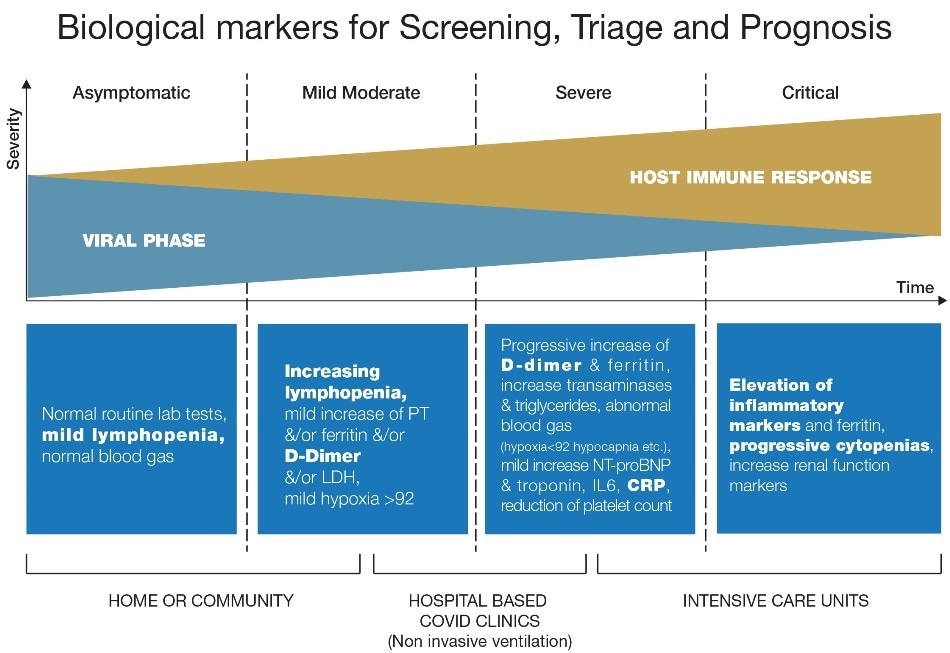
Biological markers for screening, triage and prognosis of COVID-19 – these are useful for assessing the four different levels of clinical severity. Image Credit: HORIBA Medical
The gold standard for confirmation of COVID-19 diagnosis is through the detection by molecular analysis of the viral genome using qPCR. However, detection of the virus is not a predictor of the severity of the disease, so the use of a comprehensive panel of easily measured biological markers with clinical observations can support screening and prompt patient management decisions.
Several biomarkers have been observed to be abnormal in COVID-19 infected patients and the relevance of identifying them lies in decreasing the possibility of misdiagnosing severe COVID-19 and providing more insightful information for better management of COVID-19 patients. Many cohorts of different populations have been reported, principally from China, showing abnormal laboratory assessments of complete blood count, liver and renal function, biochemical and coagulation testing and inflammatory factors, amongst others.
The biological markers that can support COVID-19 screening, triage and prognosis discussed in the white paper can all be measured on routine hematology analyzers. These markers are white blood cells (WBC), platelets, D-dimer, C-Reactive Protein (CRP) and fibrinogen; the paper provides a literature review and overview of the response of each to COVID-19 clinical severity. For example, lymphopenia and increases in CRP plus cytokines, D-dimer and fibrinogen have all been noted in a number of scientific papers to correlate with COVID-19 disease severity.
All these markers could aid in triage to help better identify COVID-19, particularly those patients most likely to require intervention and/or intensive care. They can also facilitate the continuation of other critical clinical services by aiding the separation of COVID-19 patients. Where possible, they can even be used for testing in the community to avoid the unnecessary travel of vulnerable or possibly infected individuals.
Supporting the new white paper, HORIBA Medical has also published a series of three Focus Notes which review ‘How biological markers could contribute to the monitoring of COVID-19?’. Each focuses on a different panel of biomarkers, with the first discussing hematology biological markers, the second highlighting the inflammatory marker CRP and the third detailing the use of hemostasis markers. These, as well as the white paper, are all available for download at HORIBA Medical’s Coronavirus (COVID-19) dedicated web page.
As experts in hematology, HORIBA Medical designs and manufactures a broad portfolio of systems capable of measuring a range of potential COVID-19 biomarkers, both at the point-of-care and in the laboratory. For example, in addition to Full Blood Count with WBC differentials, lymphocytes and platelets available on all of its hematology analyzers, HORIBA combines these parameters with CRP on its Microsemi CRP and Pentra MS CRP analyzers. The Microsemi CRP is unique as it can deliver these parameters at the point-of-care to support rapid triage and clinical decisions. Completing the portfolio, HORIBA’s Yumizen G range of coagulation analyzers provide accurate measurement of fibrinogen and D-dimer.