Researchers at the Karolinska University Hospital and University Hospital of Wales report that people who have recovered from asymptomatic or mild cases of coronavirus disease 2019 (COVID-19) may have long-term T-cell immunity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Importantly, some convalescent individuals exhibited SARS-CoV-2-specific T cell immunity in the absence of antibodies against the virus, which points to a previously unanticipated type of population-level immunity.
A pre-print version of the paper is available on the server bioRxiv*, while the article undergoes peer review.
.jpg)
Novel Coronavirus SARS-CoV-2 Colorized scanning electron micrograph of a cell (green) infected with SARS-COV-2 virus particles (purple), isolated from a patient sample. Image captured at the NIAID Integrated Research Facility (IRF) in Fort Detrick, Maryland. Credit: NIAID

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Questions surrounding SARS-CoV-2 exposure and adaptive immunity
Since the emergence of COVID-19 in Wuhan, China, late last year, the outbreak has become the largest public health emergency to affect the globe in decades.
The disease generally only causes severe symptoms in older individuals and those with underlying health conditions, where excessive inflammation can lead to difficulty breathing, respiratory failure, and death.
However, most infected individuals are either asymptomatic or only experience mild disease.
Given that the pandemic is ongoing and no protective vaccine is available, “it will be critical to determine if exposed or infected people, especially those with asymptomatic or very mild forms of the disease who likely act inadvertently as the major transmitters, develop a robust adaptive immunity against SARS-CoV-2,” write Marcus Buggert (Karolinska University Hospital) and colleagues.
Studies have indicated protection following exposure
Researchers are currently trying to map the determinants of immunity against the virus, and a study of rhesus macaques recently showed that SARS-CoV-2 infection generated near-complete protection against re-challenge in the animals. There is also limited evidence of people with previously documented COVID-19 becoming re-infected.
“Further work is therefore required to define the mechanisms that underlie these observations and evaluate the durability of protective immune responses elicited by primary infection with SARS-CoV-2,” say Buggert and team.
Most previous studies of immunity have focused on humoral responses and the generation of neutralizing antibodies. However, not all infected individuals develop antibody responses, particularly those who experience less severe disease.
Studies of SARS-CoV-1, which caused the 2002 SARS outbreak, have demonstrated that while memory B cell responses post-infection tend to be short-lived, memory T cell responses can be long-lasting.
Furthermore, researchers have detected SARS-CoV-2-specific T cells in cases of human infection.
“SARS-CoV-2-specific memory T cells will likely prove critical for long-term immune protection against COVID-19,” say the researchers. “It has nonetheless remained unclear to what extent various features of the T cell immune response associate with antibody responses and the clinical course of acute and convalescent COVID-19.”
Now, Buggert and colleagues have mapped cellular and humoral immune responses to SARS-CoV-2 among people with acute COVID-19, people who had recovered from asymptomatic, mild or severe disease, family members who had been exposed to the virus and healthy donors who gave blood either before or during the pandemic.
T-cell responses in the acute and convalescent phases
The team reports that SARS-CoV-2-specific T cells displaying highly cytotoxic activity were a hallmark of acute disease and that the level of CD38 expression correlated with disease severity.
“Equivalent functional profiles have been observed early after immunization with successful vaccines,” say the researchers.
In the convalescent phase, SARS-CoV-2-specific T cells acquired an early differentiated, stem-like memory phenotype, and subjects still had robust memory T cell responses months after infection, even in the absence of SARs-CoV-2 specific antibodies.
Buggert and colleagues say this points towards a “previously unanticipated degree of population-level immunity against COVID-19.”
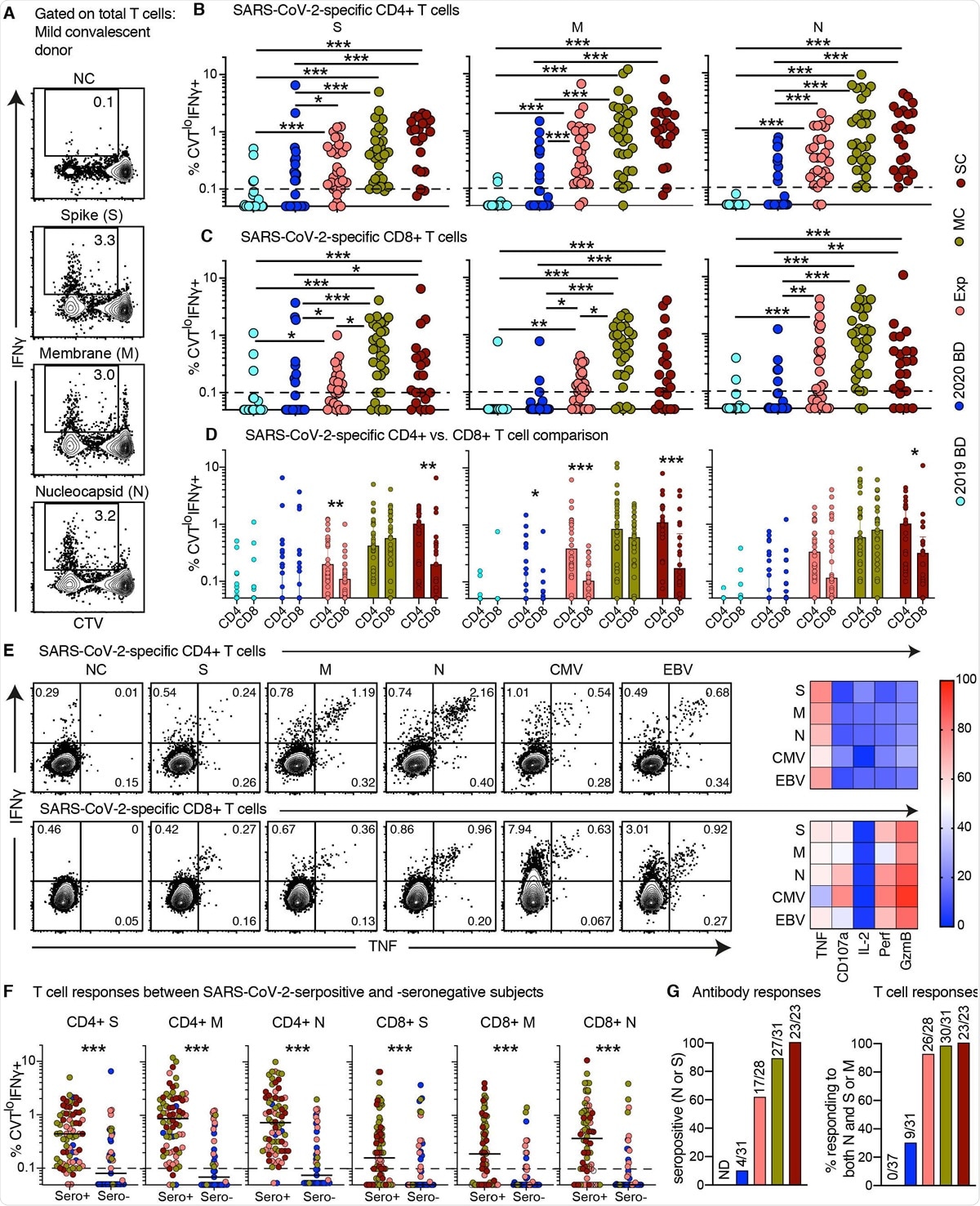
Proliferative capabilities of SARS-CoV-2-specific T cells in convalescent COVID-19. (A) Representative flow cytometry plots showing the proliferation (CTV−) and functionality (IFN-g+) of SARS-CoV-2-specific T cells from a convalescent individual (group MC) after stimulation with overlapping peptides spanning the immunogenic domains of the SARS-CoV-2 membrane (M), nucleocapsid (N), and spike proteins (S). Numbers indicate percentages in the drawn gates. (B and C) Dot plots showing the frequencies of CTV− IFN-g+ SARS-CoV-2-specific CD4+ (B) and CD8+ T cells (C) by group and specificity. Each dot represents one donor. The dotted line indicates the cut-off for positive responses. (D) Bar graphs comparing the frequencies of CTV− IFN-g+ SARS-CoV-2-specific CD4+ versus CD8+ T cells by group and specificity. Each dot represents one donor. Data are shown as median ± IQR. (E) Left: representative flow cytometry plots showing the production of IFN-g and TNF among CTV− virus-specific CD4+ (top) and CD8+ T cells (bottom) from a convalescent individual (group MC). Numbers indicate percentages in the drawn gates. Right: heatmaps summarizing the functional profiles of CTV− IFN-g+ virus-specific CD4+ (top) and CD8+ T cells (bottom). Data are shown as mean frequencies (key). (F) Dot plots showing the frequencies of CTV− IFN-g+ SARS-CoV-2-specific CD4+ and CD8+ T cells by group, serostatus, and specificity. Each dot represents one donor. The dotted line indicates the cut-off for positive responses. Key as in B. (G) Left: bar graph showing percent seropositivity by group. Right: bar graph showing the percentage of individuals in each group with detectable T cell responses directed against both the internal (N) and surface antigens (M and/or S) of SARS-CoV-2. *P < 0.05, **P < 0.01, ***P < 0.001.
The degree of population-level immunity may have been underestimated
“Almost twice as many exposed family members and healthy individuals who donated blood during the pandemic generated memory T cell responses versus antibody responses,” writes the team… “implying that seroprevalence as an indicator has underestimated the extent of population-level immunity against SARS-CoV-2.”
Buggert and colleagues say whether these robust memory T cell responses can protect against recurrent SARS-CoV-2 in the absence of virus-specific antibodies still need to be determined.
However, this scenario has previously been observed for SARS-CoV-1, and in the current study, none of the convalescent individuals had experienced any recurrence of COVID-19.
“Our collective dataset shows that SARS-CoV-2 elicits robust memory T cell responses akin to those observed in the context of successful vaccines, suggesting that natural exposure or infection may prevent recurrent episodes of severe COVID-19 also in seronegative individuals,” concludes the team.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources