The COVID-19 pandemic has spread across the whole world, causing over 13.75 million confirmed infections and over 589,000 deaths until now. Many broad-spectrum antiviral drugs have been tried in an attempt to arrest the spread of the pandemic. But no specific vaccines or therapies have been found so far, which means that only supportive management has been the mainstay.
A new paper by researchers from the USA and China and published on the preprint server bioRxiv* in July 2020 discusses a new drug based on the catalase enzyme, which could play a vital part in reducing the inflammation associated with progressive and severe COVID-19 disease. Even though the mechanism of disease is not clear as yet, the available evidence points to the occurrence of cytokine storm syndrome.
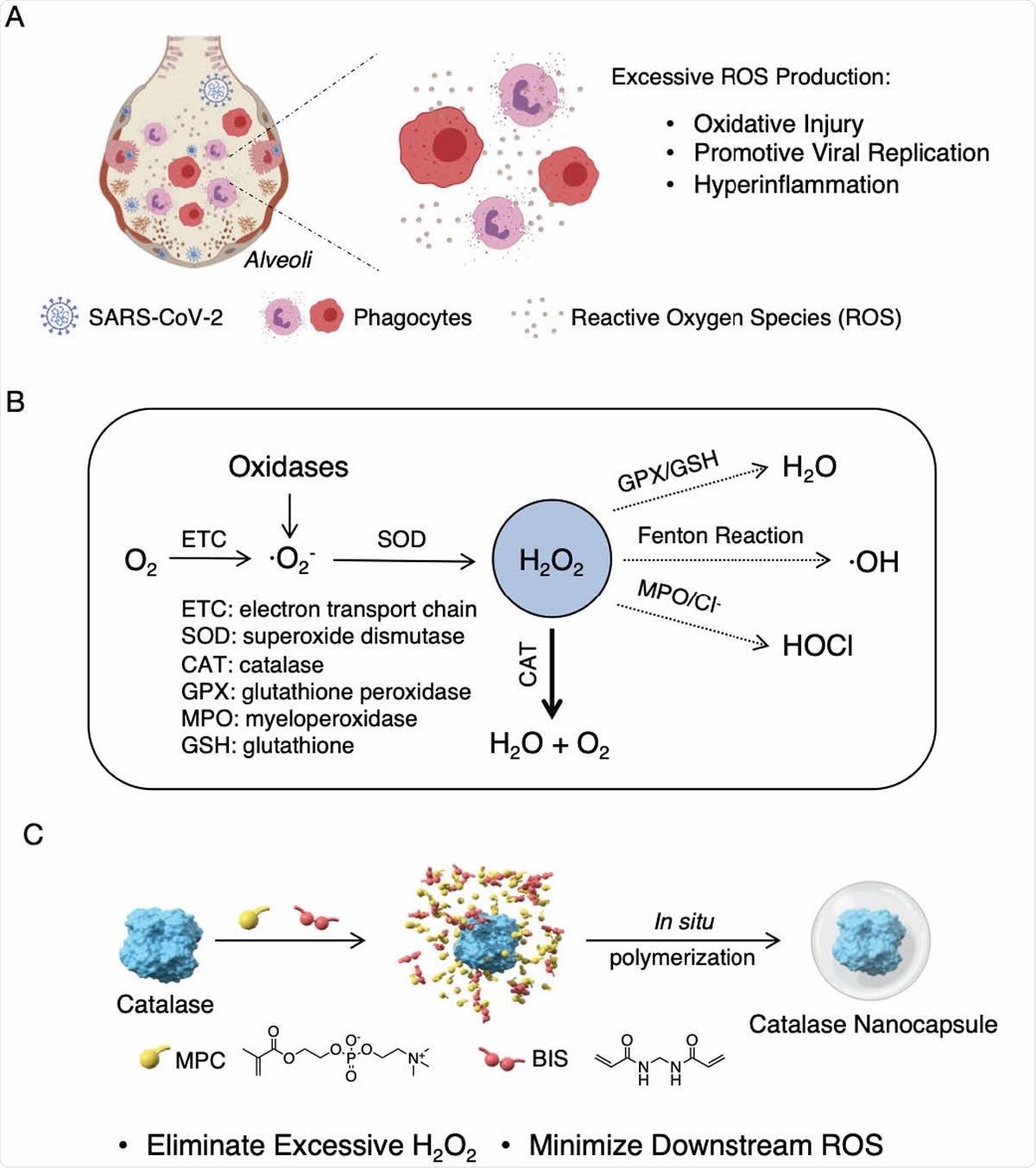
Proposed mechanism of action and synthesis of catalase nanocapsules. (A) A schematic illustrating that an elevated level of ROS causes oxidative injury, promotes viral replication, and triggers cytokine storm syndrome in COVID-19 patients. (B) The reaction pathways of ROS, suggesting that eliminating H2O2 is the key to minimizing the formation of downstream ROS. (C) The synthesis of catalase nanocapsules by in situ polymerization of MPC and BIS around individual catalase molecules exhibiting improved stability and circulation half life.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
What is Cytokine Storm Syndrome?
In some patients or following treatment with certain drugs, there is a phenomenon characterized by severe multiorgan damage caused by cytokine overproduction. Some conditions in which this occurs include organ transplants, autoimmune disease, cancer immunotherapy, and viral infections. It may be fatal in some situations if not managed properly and is called the cytokine storm syndrome.
The immediate management consists of reducing the excessive levels of inflammation and uncontrolled immune response. This involves immunosuppression as with steroids, intravenous immunoglobulin, and specific drugs targeting one or other of the cytokines involved, such as tocilizumab, an IL-6 blocker.
The Study: Reducing ROS Production
However, the current study took another route, looking at the possibility of intervening at the second level, that is, muting the production of reactive oxygen species (ROS) that correlates with inflammation, organ damage due to oxidative injury to membrane lipids, DNA and protein oxidation, inducing cell apoptosis. Higher ROS levels are also linked to higher levels of viral infection and replication.
The researchers tested the use of a drug that can regulate the level of ROS. ROS are oxygen metabolites that are potent oxidants, mostly generated by the electron transport chain in the mitochondria and cytochrome P450, but also via oxidase enzymes found in many cells, especially endothelium and phagocytes.
ROS Generation
The process of ROS generation begins with superoxide anions, which being unstable are rapidly converted to H2O2 or hydrogen peroxide, via superoxide dismutase. This may be converted to oxygen and water through the enzyme catalase, or to HOCl via myeloperoxidase (MPO), or to water through glutathione/glutathione peroxidase complex (GSH/GPX).
If the antioxidant enzymes are deficient, or if ROS are produced in excessive amounts, H2O2 may accumulate in the tissues, causing oxidative protein damage and producing more ROS. Thus, it is vital to get rid of this chemical when it is present in large amounts.
Conversely, ROS is part of the body’s weaponry against infections, and also an essential part of the body’s signaling mechanism. Its generation is essential to recruiting leukocytes to wounds, to modulate the immune response. Thus, it is needful to mitigate the excessive production of ROS rather than suppress them altogether. This could also restore immune function to normal.
The Role of Catalase
Catalase is the most abundant and effective enzyme to break down H2O2. It is found in the liver, red cells, and the alveolar cells of the lung. It is able to decompose 107 molecules of H2O2 within a second. The problem is that this enzyme is unstable.
Stabilizing Catalase
To be used as a therapeutic, catalase has to be stabilized. To do this, the researchers enclosed catalase in a thin polymer shell via in situ polymerization, using the monomers 2-methacryloyloxyethyl phosphorylcholine (MPC) and N-(3-aminopropyl) methacrylamide hydrochloride (APM), and N,N’-methylenebisacrylamide (BIS) as the crosslinker.
The polymerization surrounds each individual molecule of catalase, forming nanocapsules or n(CAT). The thin shell structure prevents the enzyme from breaking down but allows H2O2 to pass through readily. Thus, the n(CAT) is supplied in a highly active stage, with excellent stability and a longer half-life.
In fact, the n(CAT) has better thermal stability, retaining 90% of stability after incubation in buffer at 37oC compared to the native enzyme, and 87% after incubation with trypsin, 100% after storage at 4oC or 25oC, and more than 90% after freeze-drying.
Protection Against Oxidative Injury
The researchers wanted to examine the ability of this drug to prevent oxidative injury in lung tissues, using human lung alveolar epithelial cells. They cultured these cells with n(CAT) at different concentrations and found that they remained viable, ruling out any cytotoxicity.
They then cultured the cells with n(CAT) followed by the addition of H2O2, when 100% viability was observed after 12 hours. This indicates the protective ability against oxidative injury. In the third step, they cultured the cells with H2O2 and then added n(CAT) to the injured cell culture. The viability, which had dropped to 50% improved to 73%, showing its ability to help damaged cells to regenerate.
Regulating the Immune Response
The next part of the experiment was aimed at studying the ability of n(CAT) to regulate cytokine production since excessive cytokine secretion by activated leukocytes is instrumental in inducing hyperinflammation in severe COVID-19. When the white cells were cultured with lipopolysaccharide alone, without n(CAT), the production of TNF-a and IL-10 was significantly higher than when they were cultured with n(CAT). In the latter situation, the cytokine levels were similar to those found with non-activated cells. Thus, this enzyme can act as an immunoregulator as well.
Protection Against Leukocyte-Induced Epithelial Injury
When white cells and H2O2-injured alveolar cells were co-cultured, the viability dropped from 85% to 71%. Adding in n(CAT) restored viability in a dose-dependent manner, from 82% to 91% at doses of 8 to 40 μg/mL. When co-cultured with activated leukocytes, the alveolar epithelial cells showed only 67% viability, but this increased with the addition of n(CAT) in a dose-dependent manner, from 78% to 91%. Thus, n(CAT) protects the alveolar cells from injury by activated leukocytes.
The researchers also found that n(CAT) persists in circulation for a longer duration than the native enzyme. When administered intratracheally by nebulization, it is retained mainly within the lung.
Suppression of Viral Loads
What was the effect of n(CAT) on viral loads? The researchers observed that viral load in rhesus monkeys exposed to the virus intranasally fell rapidly after two days, but in one animal, it fell in just one day. The same was seen with intravenous administration of the virus. Thus, n(CAT) was able to suppress viral replication in rhesus monkeys. However, it does not cause toxic changes in the liver or kidney.
Benefits to the use of n(CAT)
There are several advantages to the use of catalase in the therapy of severe COVID-19. It has anti-inflammatory and protective effects on alveolar epithelium cells in animal models. It is noticeably safe. It is already in use as a food additive and dietary supplement. Its manufacture is feasible, as shown by a pilot project.
Thus, the researchers say, “In contrast to the current focus on vaccines and antiviral drugs, this may provide an effective therapeutic solution for the pandemic, as well as treatment of hyperinflammation in general.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Qin, M. et al. (2020). An Antioxidant Enzyme Therapeutic for COVID-19. bioRxiv preprint. doi: https://doi.org/10.1101/2020.07.15.205211. https://www.biorxiv.org/content/10.1101/2020.07.15.205211v1
- Peer reviewed and published scientific report.
Qin, Meng, Zheng Cao, Jing Wen, Qingsong Yu, Chaoyong Liu, Fang Wang, Jianjun Zhang, et al. 2020. “An Antioxidant Enzyme Therapeutic for COVID-19.” Advanced Materials (Deerfield Beach, Fla.) 32 (43): e2004901. https://doi.org/10.1002/adma.202004901. https://onlinelibrary.wiley.com/doi/10.1002/adma.202004901.