With the ongoing COVID-19 pandemic continuing to spread to hundreds of thousands the world over, scientists are still seeking an effective treatment or vaccine. Now, a new study published on the preprint server medRxiv* in July 2020 reports that an older treatment modality, namely, the use of convalescent antibody-rich plasma, brings down the mortality rate in hospitalized COVID-19 patients by an astonishing 57%. This adds evidence for the use of convalescent plasma in the treatment of high-risk patients.
It has been over a hundred years since convalescent was first used as a source of passive immunity. The antibodies produced against a specific pathogen are of great use in treating outbreaks caused by it. Despite the lack of clinical evidence for its efficacy in COVID-19 disease, there are multiple randomized controlled trials (RCTs), case-control studies, and case series dealing with its use in this situation.
These are mostly too small to provide adequate proof or did not run until the endpoint. In the absence of large randomized controlled trials, support for its effectiveness is lacking, causing doctors to disagree on whether it should be routinely used in hospitalized patients.
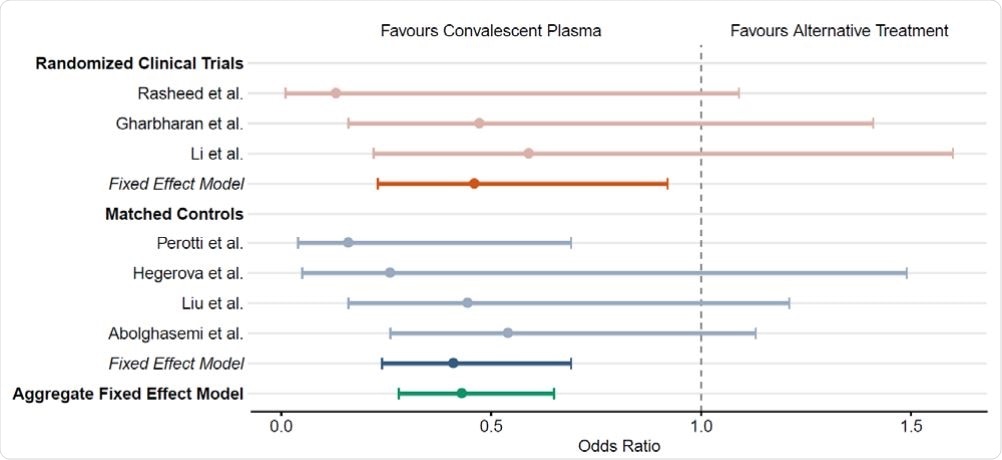
The impact of human convalescent plasma therapy on COVID-19 patient mortality. Forest plot illustrating odds ratios (OR) and 95% confidence intervals for controlled studies and aggregate fixed-effect models. Randomized clinical trials including Rasheed et al.10, Gharbharan et al.8 , and Li et al.7 are represented in orange. Matched controlled studies including Perotti et al.13, Hegerova et al.11, Liu et al.12, and Abolghasemi et al.14 are represented in blue. Aggregate fixed-effect models for each study type are represented by shaded hues. The overall aggregate fixed effect model is represented in teal.
Meta-Analysis of Prior Studies
The researchers pooled the data from different types of trials, whether RCTs (12), case-control studies (5), or case series (4), to obtain an aggregate estimate of the calculated mortality rate overall.
They included 804 patients in all, aged 48 to 70 years, with a higher proportion of men in most studies. Most of the patients included had severe or critical COVID-19 illness and had received plasma transfusion. At the time of transfusion, up to ~80% of patients had been put on mechanical ventilation. Patients were followed up from 7 to 30 days.
Dramatic Drop in Mortality
The researchers found that the mortality rates were comparatively low, in all the four case series, from none to 13%. Among the RCTs too, the rates were lower at 13% among the transfused patients compared to 26% among the non-transfused. The difference was obvious in the case-control studies, at 12%, just half of the 25% recorded in the non-transfused controls.
Thus, the odds of death were reduced by approximately 54% and 60%, respectively, in patients who received convalescent plasma, in the RCTs and case-control studies, respectively. The researchers adjusted for age, mechanical ventilation, and even for different periods of follow up, and found no significant difference in the reduction in mortality.
The investigators concluded that their analysis of the outcomes of patients who received convalescent plasma showed a marked reduction in mortality in this group compared to similar patients who did not. They say, “These results favor the efficacy of convalescent plasma as a COVID-19 therapeutic agent.”
Explaining the Benefit of Convalescent Plasma
The most plausible explanation for the effectiveness of this therapy is that the plasma contains neutralizing antibodies that prevent the virus from entering the host cells. Other biological mechanisms may also operate. These results, they point out, “align with similar analyses of historical data from convalescent plasma trials for viral diseases such as the 1918 flu epidemic, Severe acute respiratory syndrome, and H1N1 influenza.”
The meta-analysis is limited by its inclusion of many studies with wide variation in the country of origin, the temporal setting of the study with relation to the global course of the pandemic, the diagnostic and therapeutic norms in each patient cohort, and the antibody titer in the convalescent plasma used, as well as the plasma volume transfused. However, the fact remains that the treatment appears to robustly deliver encouraging results irrespective of the country of the study, and is not associated with significant side effects.
Encouraging Early Results
Again, many of these patients actually were transfused as a last-ditch measure, late in the course of illness. However, history shows that in many infections such as streptococcal pneumonia and bacterial meningitis, during the pre-antibiotic pre-vaccination era, the optimal time for administration of convalescent plasma was very early in the course of hospitalized illness. If so, earlier timing of convalescent plasma transfusion might well have reduced the COVID-19 mortality even further.
The researchers, therefore, sum up, “The results provide encouragement for its continued used as a therapy and may have broad implications for the treatment of COVID-19 and design of RCTs.”
The team was comprised of 21 researchers from the Mayo Clinic, Johns Hopkins School of Public Health, Michigan State University, Cooper Medical School of Rowan University, Albert Einstein College of Medicine, Icahn School of Medicine at Mount Sinai, Washington University School of Medicine in St. Louis, Johns Hopkins University, and Houston Methodist Hospital.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources