The COVID-19 crisis still threatening the world has been met so far by non-pharmaceutical interventions (NPI), including social distancing and mobility restrictions. However, these cannot be sustained indefinitely without enormous economic and social fabric breakdown. Therefore, scientists are racing to find effective vaccines and antiviral drugs to counter the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Concerning the earlier Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS) viruses, two stabilizing proline residues were introduced in the S2 subunit in the hinge loop between the CH and heptad repeat 1 (HR1). This has been shown to be the case with the SARS-CoV-2 spike protein as well, following an additional mutation was introduced at the S1/S2 interface furin cleavage site.
Designer Modifications
Several teams have attempted to create vaccines based on designer S proteins, of which three approaches are compared. These are the wildtype signal peptide (wt SP), tissue plasminogen activator signal peptide (tPA SP), and with or without stabilizing substitutions. These have been shown to induce neutralizing antibodies and confer protection against infection with SARS-CoV.
A new research paper by scientists at Janssen Vaccines & Prevention BV, in the Netherlands and published on the preprint server bioRxiv* is based on the assumption that an effective vaccine against these infections will induce neutralizing antibodies and an immune response favoring Th1 immunity. Many researchers have noted that the Th2 type of immune response elicited by some earlier prototype SARS-CoV vaccines could cause antibody-dependent enhancement in animal models. Though it is not clear whether this is a risk in humans, this theoretical risk could be mitigated by ensuring that a Th1 response is induced.
To achieve this, the current study uses vaccines based on transgenes delivered by recombinant adenovirus type 26 vectors (Ad26) incapable of replication. These are known to be safe in humans but to elicit neutralizing and binding antibodies, as well as cellular responses based on CD4 and CD8 T cells, culminating in a Th1-based response. The fact that these transgenes are suitable for scalable and industrial processes makes it a very viable option for vaccine development.
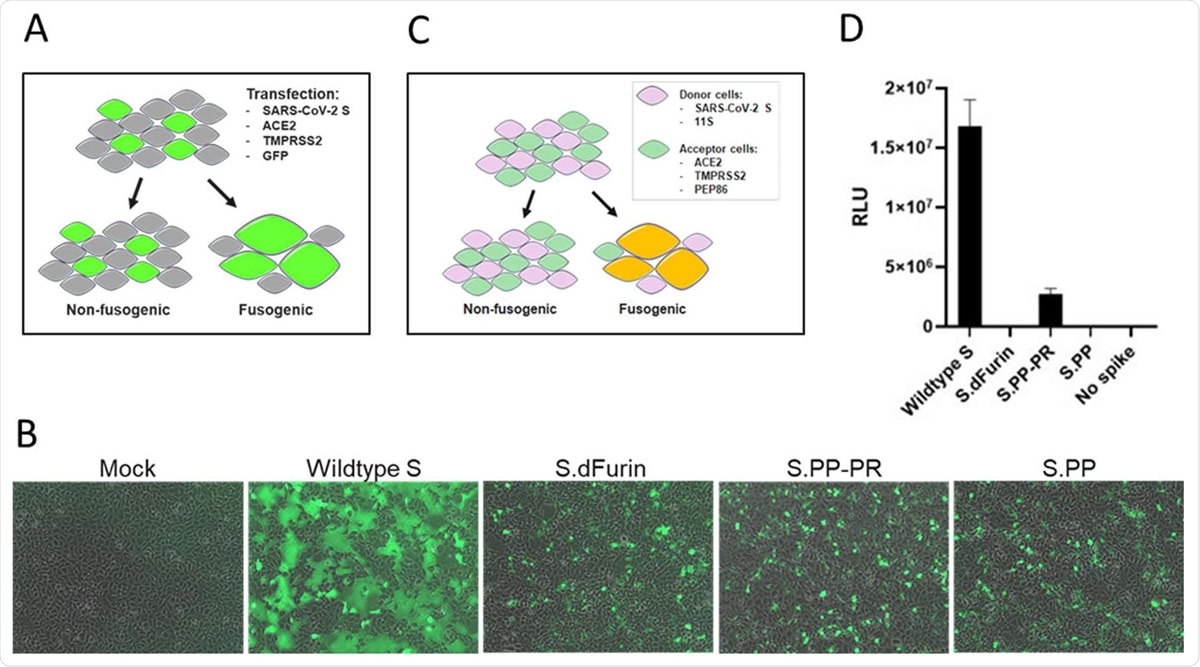
Effect of stabilizing mutations on fusogenicity of S protein. A) S protein fusogenicity as measured in a cell-cell fusion assay in HEK293 cells by co-transfection of plasmids encoding S protein, ACE2, TMPRSS2, and GFP. B) Overlay of GFP and brightfield channel 24 h after transfection, as in the setup of (A). The different S protein constructs are indicated; mock is an untransfected monolayer. C) Quantitative cell-cell fusion assay setup. D) Luciferase signal shown as relative light units (RLU) measured at 4 h post mixing of donor and acceptor cells, as in the setup of (C).

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Designing the Immunogen
The researchers generated plasmids encoding seven different full-length variants of the spike protein, with each of the above substitutions or mutations in various combinations or alone. They then assessed the antigenicity of the spike protein, finding that the highest binding to ACE2 and to neutralizing monoclonal antibodies was to the spike protein constructs that contain the double proline or furin cleavage site mutations. When stabilizing substitutions were not present, the ACE2 binding decreased, perhaps because the S1 domain was shed. This shedding may occur in the absence of ACE2 as well if the spike protein is not stabilized.
Higher Immunogenicity
The highest neutralizing to non-neutralizing antibody binding was seen with the construct that contained wt SP along with stabilizing mutations, namely, the double proline substitutions and the furin cleavage site mutations. This shows that S is expressed at high levels in the prefusion spike protein conformation, or if only low levels of S1 are shed.
The mature S proteins showed correct cleavage at the N-terminal ends with the wt SP, whereas that with the tPA SP showed lower yields of the trimer and lower stability. This is probably the reason for the lower neutralizing antibody binding with the tPA.S variant compared to native S.
They also found that the presence of the double proline substitutions and the furin cleavage site mutations, either in isolation or together, was enough to inhibit syncytium formation in a cell-cell fusion assay. The presence of wt SP produced high levels of fused cells in this assay. The double proline substitutions, and the furin cleavage site mutations, alone or in combination, caused the protein to become non-fusogenic, indicating that these changes prevent its conformational change to the post-fusion protein and increase its immunogenicity.
Not only did the substitutions cause a change in the spike protein affinity, but the stabilizing double proline mutations increased the immunogenicity. Mice that received one intramuscular immunization of the Ad26.S.PP generated higher titers of binding antibodies and neutralizing antibodies in a dose-dependent manner compared to the Ad26.S transgene.
Th1-Skewing of Immune Response
The Ad26.S also induced a Th1-skewed immune response, as judged by the high secretion of the Th1 hallmark cytokine IFN-γ relative to IL-4, IL-5, and IL-10. Again, the IgG2a secretion was increased following exposure to compared to IgG1, which indicates Th1-skewing as the latter is produced indiscriminately in Th1 or Th2 responses but the latter only in Th1-predominant immune reactions.
Implications
This side-by-side comparison of different S protein designs showed that the wt SP peptide is appropriate for correct cleavage and subsequent correct protein folding. This is required for the increased production of anti-S binding antibodies and neutralizing antibodies.
This AD26.S.PP is already known to induce protective antibodies against the infection in a nonhuman primate model, by earlier research.
The study sums up, “Ad26.S.PP, from now on named Ad26.COV2.S, was identified as our lead SARS-CoV-2 vaccine candidate and is currently being evaluated in a phase I clinical trial.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources