As the COVID-19 pandemic is still rampant, effective antiviral drugs are urgently needed to aid in tackling this disease caused by SARS-CoV-2, but also possibly emerging outbreaks caused by other coronaviruses in the future.
One of the best-characterized, but possibly underutilized drug targets for coronaviruses is the main protease (Mpro or 3CL protease). More specifically, it belongs in a class of homologous cysteine proteases required for viral replication in diseases such as the original SARS, MERS, and now also COVID-19.
The advantage is that the amino acid sequence of SARS-CoV-2 Mpro is 96% identical to the SARS-CoV Mpro amino acid sequence, while their three-dimensional structures also show striking similarities. This means that we can readily exploit our knowledge from the SARS outbreak in 2002 and 2003 for the current pandemic.
Moreover, there are no known human proteases that exhibit similar cleavage specificity as Mpro, suggesting that the development of inhibitors that specifically target this protease and are not coupled with off-target toxicity is a viable option.
And while repurposing SARS-CoV Mpro inhibitors to target the SARS-CoV-2 has already been tried, additional Mpro inhibitors will be needed – either to improve their properties or to use them in combination treatments.
In this seminal paper, the researchers from the Department of Chemistry and Department of Pharmaceutical Sciences at the University of California in Irvine, US, describe the design and preliminary evaluation of UCI-1 (University of California, Irvine Coronavirus Inhibitor-1) with rather promising results.
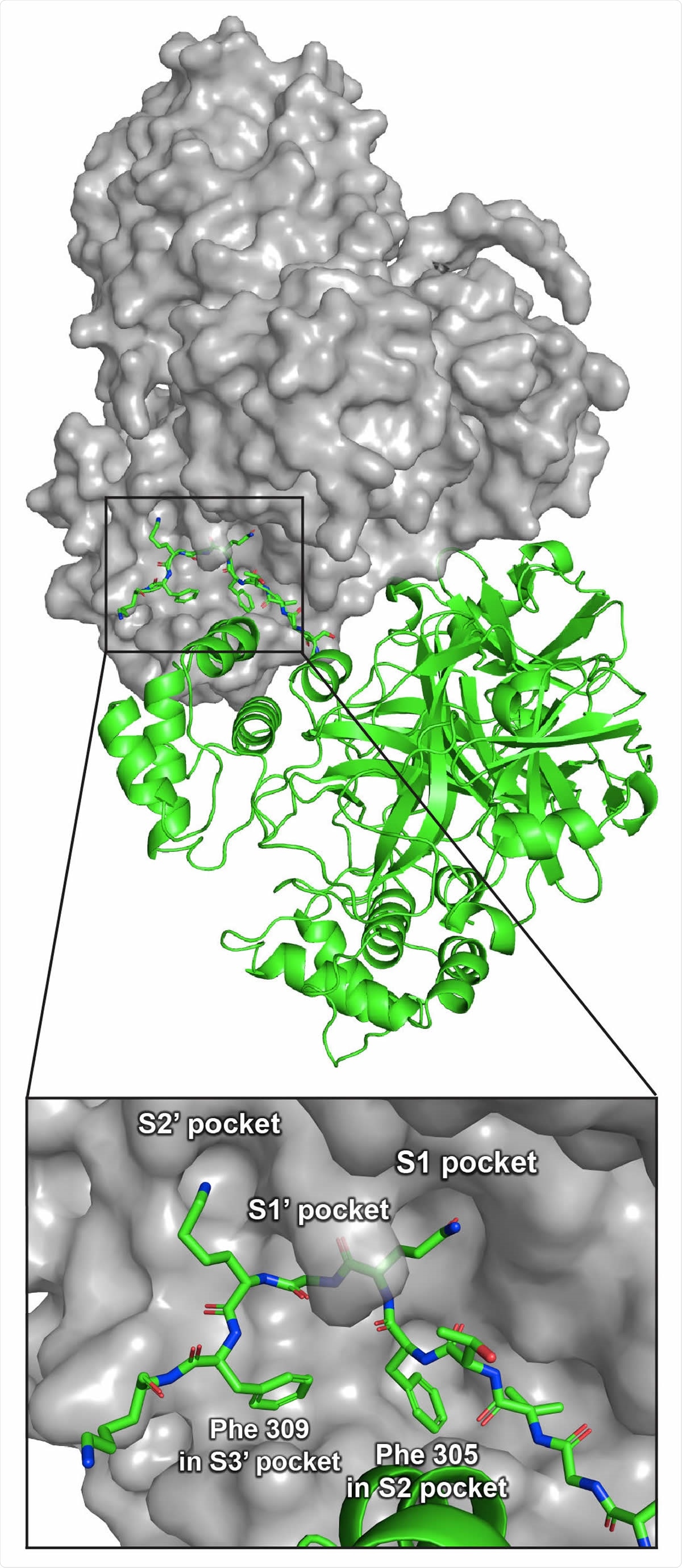
Crystal structure of Mpro316 showing two Mpro 316 dimers in two adjacent asymmetric units (PDB 5B6O). One dimer is shown in grey surface view; the other dimer is shown in green cartoons. The inset shows a detailed view of C-terminal residues 301–310 of the C-terminal autolytic cleavage site of one Mpro 316 molecule in the active site of another Mpro 316 molecule.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The design and appraisal of the cyclic peptide inhibitor
In a nutshell, UCI-1 was designed to mimic the conformation of a C-terminal autolytic cleavage site of the SARS-CoV Mpro, which represents a naturally occurring Mpro substrate.
To achieve the aforementioned design, the researchers have utilized the molecular visualization software PyMOL (i.e., an open-source molecular visualization system) to construct an authentic model of the envisioned cyclic peptide.
Furthermore, to evaluate the inhibitory properties of UCI-1 against Mpro, this research group used an already established fluorescence-based Mpro inhibition assay kit. Enzyme inhibition assay was additionally analyzed with liquid chromatography-mass spectrometry (LC–MS).
Finally, to appraise whether UCI-1 is cytotoxic, they have exposed human embryonic kidney cells to varying concentrations of UCI-1 for 72 hours and then evaluated cell death with the use of a lactase dehydrogenase assay.
Non-toxic and active at mid-micromolar concentrations
"The design and preliminary evaluation of UCI-1 demonstrate that cyclic peptides that mimic the conformation of linear peptide substrates of Mpro can be developed as inhibitors against Mpro", claim study authors in their bioRxiv paper.
More specifically, the use of in vitro Mpro inhibition assay revealed that UCI-1 is active against SARS-CoV-2 Mpro at mid-micromolar concentrations. Additional liquid chromatography-mass spectrometry (LC-MS) analysis has shown that UCI-1 resists cleavage by Mpro, despite containing a scissile amide bond (i.e., a covalent bond amenable to enzyme breakage).
But even more importantly, UCI-1 is found to be non-toxic toward human embryonic kidney cells at concentrations that inhibit Mpro, which gives hope that the compound could be optimized for human use.
Promising drug candidates against COVID-19
While the activity of UCI-1 is somewhat modest when compared to other known Mpro inhibitors, its preliminary evaluation lays the foundation for the development of additional cyclic peptide inhibitor analogs of UCI-1 with enhanced activity against Mpro.
"Design and development of next-generation UCI-1 analogs will likely produce better inhibitors", emphasize study authors. "Due to the urgency of COVID-19, we elected to share our initial hit, UCI-1, in this preprint", they add.
The authors hope that this will encourage other researchers to begin thinking about cyclic peptide inhibitors as promising drug candidates in our fight against COVID-19. And indeed, next-generation analogs of UCI-1 with enhanced activity against Mpro may be a magic bullet that we desperately need.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources