During the 2002-2004 severe acute respiratory syndrome coronavirus (SARS-CoV-1) outbreak, cats living with patients were found to be infected and to transmit the virus. With the current COVID-19 pandemic caused by the similar severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), researchers from Kansas State University, Louisiana State University, and the Icahn School of Medicine at Mount Sinai have been looking into the possibility that the virus is also transmitted by domestic pets, especially cats.
Prior research has shown that under both natural and experimental conditions, the virus infects several species, including natural spread to dogs, cats, lions, and tigers, and farmed mink. The experimental spread has been demonstrated in dogs, cats, ferrets, pigs, chickens, and ducks with efficiency in most species except in dogs, pigs, and birds. Nonhuman primates show the capability to be infected with the virus resulting in mild to a severe spectrum of disease.
The current study is aimed at understanding how animals living at home can play a role in maintaining the live virus and spreading it among other animals and humans, as well as to understand which host species are naturally vulnerable to the infection.
Infection of Cats Remains Asymptomatic
The study showed that though domestic cats became infected after exposure to intranasal and oral inocula of the virus, and spread the infection to their contacts, they showed no clinical signs of infection other than a temperature over 38.7 oC on day 2. The sentinel cats who were exposed to the infected cats on day 1 after the inoculation of the latter showed higher temperatures on days 1, 10, and 12 following contact, but not otherwise.
All the animals in the study showed a steady body weight, indicating normal health. There were no marked alterations in the blood cells or serum biochemistry, and the white cell counts were normal for the most part.
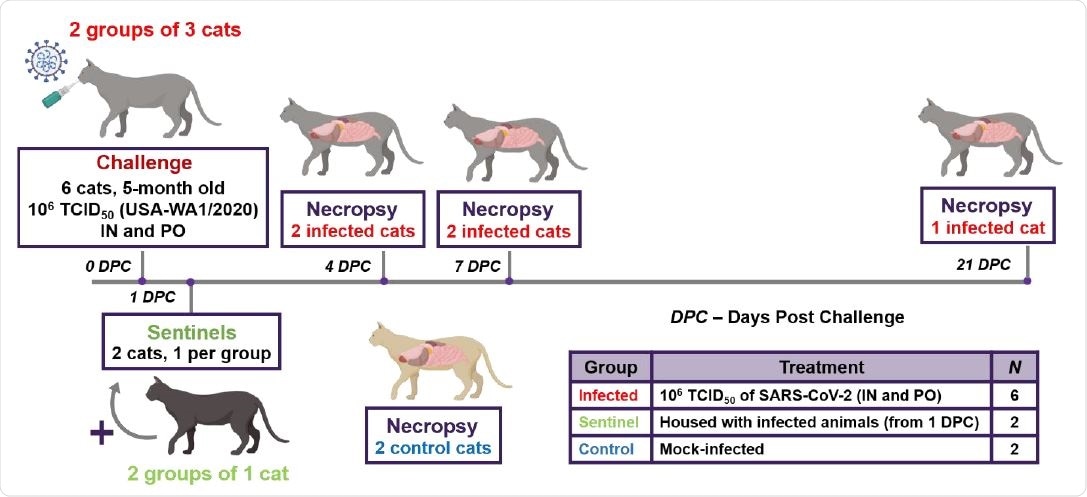
Study design. Ten cats were placed into three groups. Group 1 (principal infected animals) consisted of six cats (three cats/cage) and was inoculated via intranasal (IN) and oral (PO) routes simultaneously with a total dose of 1 x 106 TCID50 of SARS-CoV-2 in 2 ml DMEM. The cats in Group 2 (n=2; sentinel contact animals) and Group 3 (n=2; mock control animals) were housed in a separate room. At 1-day post challenge (DPC), the two cats in Group 2 were co-mingled with the principal infected animals in Group 1 (one cat per cage) and served as sentinel contact controls. The remaining two cats in Group 3 remained housed in a separate room and served as mock-infected negative controls. Principal infected animals were euthanized and necropsied at 4 (n=2), 7 (n=2) and 21 (n=1) DPC to evaluate the course of disease. The two negative control animals in Group 3 were euthanized and necropsied at 3 DPC. The remaining three animals from Group 1 (one principal infected animal) and Group 2 (two sentinel contact animals) were maintained for future re-infection studies.
Virus Present in Upper Airway
When the animals were tested via nasal swabs for the presence of viral RNA, all the infected cats tested positive from days 1 to 10, but the maximum at days 1 through 5 following contact. As for the contact animals, they tested positive from the second day from contact, remaining so up to the ninth day. The highest copy number was on day 6 or 7, comparable in magnitude to that observed in the infected animals at days 1 through 5 after inoculation.
Oropharyngeal swabs became positive and remained so from days 1 through 10 post-contact, for the infected animals, and from days 2 to 4 for the contacts. The peak copy number was on day 4 from inoculation and day 4 post-contact, respectively, but was 1-2 logs lower than that of the nasal swabs except for the infected cats on day 4 post-inoculation.
Virus Found in Respiratory Tissues
Respiratory tract tissues showed the presence of the virus at day 4 and 7 post-inoculation with 107 to 1011 copies/mL in all the autopsied cats, but at lower levels in the lungs relative to the nose and mouth, or upper airway, on day 7. It continued to be found in bronchial and lung samples at 21 days from inoculation, and in the nasal wash and bronchioalveolar lavage fluid (BALF) obtained on days 4 and 7, but not day 21. Lung parenchyma was consistently negative for viral antigens and RNA at all these time points.
Virus Present in Other Organs
The researchers found viral RNA at high levels in rectal swabs from day 3 of inoculation and day 2 after contact with the infected cats, continuing to be detected at up to day 14 or 13 from inoculation and contact, respectively. The animals became negative on day 21 from inoculation of the primary infected cats.
Other sites of infection included the gut, tonsils and spleens, other lymph nodes, heart, kidneys, olfactory bulbs, and bone marrow from day 4 onward at least up to day 21. Copy number was highest in the tonsils, and the lymph nodes of the mesentery, as well as the trachea and bronchi, on all days, while spleen positivity was observed only after day 4 of inoculation. The gut and heart tissue were pooled in all animals and showed a uniformly positive reaction for viral RNA.
The cerebrospinal fluid showed evidence of involvement in some but not all cats at day 7 but not 21 following inoculation, while all blood samples remained negative for the viral PCR. Tissue samples showed signs of inflammation, all these showing productive infection. In other words, the early presence of the virus and viral persistence was seen only in the upper respiratory tract and in lymphoid tissue.
In contrast, early infection was absent in the spleen, which showed test positivity only on day 7 and day 21.
Seroconversion of Cats Following Infection
All infected and contact cats showed seroconversion on days 10, 14 and 21 from inoculation, and the antibody titers for neutralizing antibodies were from 1:40 to 1:320. Neutralizing antibodies were not found in the infected and contact animals before the seventh or tenth day, respectively, while anti-N antibodies were present from day 7 and day 10 from inoculation, respectively. Anti-receptor binding domain (RBD) antibodies were found in infected and contact animals from day 7 and day 14 from inoculation, respectively, through 21 days.
The presence of neutralizing antibodies with the absence of clinical and histologic signs of severe infection indicates successful recovery via an antibody-mediated immune response within 3 weeks of experimental infection. Spread from the infected to the contact animals occurred within 2 days of contact, as found in earlier reports. The most probable routes are the respiratory and gastrointestinal tracts, as indicated by the high viral shedding. The airborne spread is considered improbable as an efficient route of spread among cats, on the basis of earlier studies.
Conclusion and Future Research
The researchers say these findings confirm the susceptibility of cats, in particular, to this infection and point to them as potential reservoirs of the virus. Cats older than about 4 months rarely become sick as a result of the infection, despite high levels of viral RNA in a wide variety of tissues at day 7. In most cases, the virus was cleared by 3 weeks.
The virus was detected in the upper airway, lymph nodes, and CSF, agreeing with earlier findings of viral RNA and particles in the respiratory tract of young and subadult cats at 3 days from inoculation, but not by the sixth day in the latter.
Differences in viral shedding in feces from earlier studies may be due to the different ages of the cats in the other studies as well as the variation in the viral strain employed. It seems, from this study and earlier research, that juvenile cats suffer mild to moderate inflammation of the upper airway and lymph nodes.
The researchers sum up: “These findings correlate with the absence of clinically evident respiratory disease following experimental infection, with the duration and magnitude of viral shedding, and with the onset of SARS-CoV-2 specific antibody responses; and with no histologic changes or viral RNA and viral antigen present within the respiratory tissues by 21 DPC.” In response, they suggest that cats should be screened to understand the spread and natural course of the viral infection, as well as to help prevent such transmission. The methods used for such surveillance should be nasal and rectal swabs.
Cats with the infection should be treated as needed, and other cats in their proximity due to the rapid spread of the virus to the latter. Workers who have much to do with such pets need protection as well, and infected animals must be quarantined as well. Moreover, to avoid the owners from abandoning their cats, they must be told about the risks and what preventive steps they can take to avoid viral spread and illness.
Adult cats seem to be relatively immune to repeated infection and have higher titers of neutralizing antibodies than in the current study. More research is needed to help tease out the way infection takes place, its clinical impact, and the disease process, to model the virus in preclinical studies that will help design better therapies and vaccines against future pandemics.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Gaudreault, N. N. et al. (2020). SARS-CoV-2 Infection, Disease and Transmission in Domestic Cats. bioRxiv preprint. doi: https://doi.org/10.1101/2020.08.04.235002. https://www.biorxiv.org/content/10.1101/2020.08.04.235002v1
- Peer reviewed and published scientific report.
Gaudreault, Natasha N., Jessie D. Trujillo, Mariano Carossino, David A. Meekins, Igor Morozov, Daniel W. Madden, Sabarish V. Indran, et al. 2020. “SARS-CoV-2 Infection, Disease and Transmission in Domestic Cats.” Emerging Microbes & Infections 9 (1): 2322–32. https://doi.org/10.1080/22221751.2020.1833687. https://www.tandfonline.com/doi/full/10.1080/22221751.2020.1833687.