The COVID-19 pandemic has been causing thousands of cases all over the world, but testing kits and personnel are still in short supply. The technique of viral detection at present, using reverse transcriptase quantitative polymerase chain reaction (RT-qPCR) is involved, and the assay kits are costly. As a result, a new study by Yale University researchers and published on the preprint server medRxiv* in August 2020 shows that a new saliva test named SalivaDirect simplifies the test approach, avoids invasive samples collection, and the need for trained personnel to collect specimens.
SalivaDirect is a mass testing approach, which ensures greater safety during sample collection both at the time of collection and during its processing. It uses fewer reagents and thus cuts the costs. It is also more flexible in that the reagents and instruments do not need to be acquired from a specific vendor. Moreover, the assay is suitable for a high-throughput workflow and has fast turnaround times.
Saliva collection is much easier to collect, and no particular criteria have to be met for a swab and a collection vessel, cutting down on the costs of the diagnostic test. Secondly, unlike nasopharyngeal swab collection, there is no sneezing or coughing involved in obtaining the specimen, making it safer, and preventing healthcare workers from being exposed to the infection. Thirdly, it is likely to provide more consistent results with fewer false negatives as a result of the more straightforward collection methods.
The testing workflow has also been adapted for speed, reduced costs, and efficiency by leaving out the nucleic acid extraction test, which is part of most current viral RNA tests. This means that preservative-containing saliva collection tests are no longer required, nor are specialized reagents needed, or equipment for this step.
The researchers used the CDC-primer probe sets, which are among the most reliable and sensitive for virus detection but modified by removing the less sensitive probe.
The Steps
The three steps involved are the collection of saliva without preservatives, treatment with proteinase K, and heat inactivation, followed by dualplex RT-PCR testing. The viral RNA is found to be stable in saliva for seven or more days at room temperature, at 4 degrees C, and in fact, cycle threshold (Ct) values were lower, indicating higher RNA levels after storage for seven days at 30 degrees C compared to fresh specimens. However, proteinase K treatment and heat inactivation led to higher Ct values compared to nucleic acid extraction, since nucleic acid is concentrated fourfold in the extraction process. And finally, multiplex detection led to higher detection compared to singleplex detection.
The supply chain bottlenecks are considerably reduced as a result of all these adaptations, but the lower limit of detection is still acceptable at 6-12 copies/μL. Moreover, the results are highly concordant with those achieved by currently validated tests.
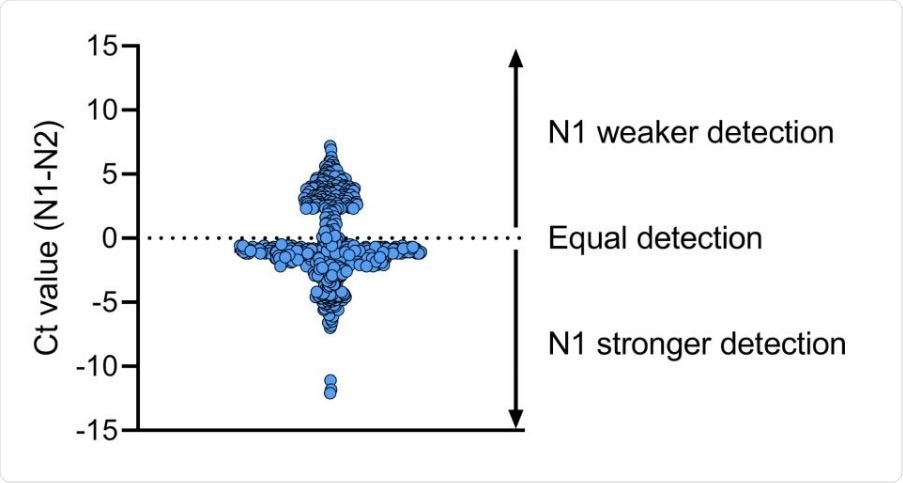
The N1 primer-probe set is more reliable than N2 for SARS-CoV-2 detection. We compared Ct values for N1 and N2 primer-probe sets for 613 clinical specimens and found that overall the N1 primer-probe set detects a stronger signal as compared to N2. Shown is the difference in Ct value between N1 and N2 and the dotted line indicates equal Ct values for N1 and N2.
High Positive and Negative Agreement
The researchers compared the Ct values for the N1 nucleoprotein of the saliva specimens with the values obtained with the modified CDC assay. While the median Ct values were slightly higher indicating weaker detection by SalivaDirect, in absolute terms, the number of false negatives was 3/41, ~7%, all of which had Ct values between 35 and 40 with the modified CDC assay.
They then compared the results obtained by the FDA-approved TaqPath combo kit using pairs of positive and negative saliva and nasopharyngeal swab specimens collected from inpatients and health workers, respectively. They found that saliva and swab samples agreed in about 84% of cases, with three samples showing different results between the pairs. The Ct values remained comparable between the sample types for each of the viral proteins tested for. Thus, saliva is a good option for testing.
The SalivaDirect test showed still higher agreement at 94% with the swabs tested by TaqPath, but with Ct values higher for SalivaDirect by a median of 3.3, probably due to skipping the nucleic acid extraction step and perhaps because of the difference in the instruments used. Three of 37 swabs were negative with TaqPath and weakly positive with the CDC assay. The saliva specimens in these pairs were positive for both the tests. Three of the nasopharyngeal tests turned out negative, but SalivaDirect returned a positive test.
When saliva specimens alone were compared between TaqPath and SalivaDirect, the agreement for positives was 97% and for negatives 100%. None of the negative saliva specimens by either test were false positives.
Affordability
By current standards, the cost of one SalivaDirect Test will be $1.29-$4.37/sample. The test has been submitted as a laboratory-developed test for approval under Emergency Use Authorization to the US FDA on July 14, 2020.
The scientists say, “SalivaDirect can help to realize large-scale testing of the general public to facilitate isolation and contact tracing of cases with the ultimate goal of preventing the spread of SARS-CoV-2.”
Future Directions
The current protocol is intended for testing saliva only and not for hospitalized patients where the saliva may be mixed with mucus or blood limiting the performance of PCR. This is one limitation, as is the requirement for electricity and RT PCR instruments. Validation must be carried out if it is to be used for mass testing with a low prevalence of infection to determine its effectiveness for the costs incurred. The researchers are carrying out more extensive studies at present to confirm further the positive and negative agreements as well as for pooled test outcomes. This could add to the single test now approved for both asymptomatic and pooled testing. Robotic handling is also being tested, as well as early pediatric testing.
The aim is to provide an easy and inexpensive test without commercializing it. The study concludes: “We encourage other groups to make their own adjustments to fit their specific needs or to improve capacity. Thus, our broad FDA EUA application provides a basis for organizations looking to use non-invasive sampling coupled with a simplified molecular testing scheme for SARS-CoV-2 surveillance.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources