In the view of recent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreak, movement restriction (lockdown) and mass testing are considered to be the most important measures to control the viral spread. In the UK and US, many websites are selling direct to user self-sampling and testing kits to detect SARS-CoV-2. Recently, a cross-sectional observational study was carried out to check the completeness and accuracy of information provided by these websites.
The study findings have revealed that users purchasing these kits online are provided with incomplete and misleading information regarding the accuracy of tests, interpretation of results, and intended use. The study by researchers at the University of Birmingham and the University of Warwick in the UK is currently available on the medRxiv* preprint server.
According to the World Health Organization (WHO), viral testing using polymerase chain reaction (PCR)-based kits is the best possible way to identify and isolate people who are infected with SARS-CoV-2. However, because of the low sensitivity (70%) of a single PCR testing, even having negative results from two consecutive PCR tests is not sufficient to exclude possible SARS-CoV-2 infection (the WHO). Besides viral testing kits that detect active infection, there are antibody testing kits to determine previous infection. However, the WHO does not recommend antibody testing kits for personal use, because it is still uncertain whether the presence of antibody ensures adaptive immunity to protect individuals from future infection.
Study objective
Direct to user kits available on different websites should be provided with high-accuracy and thorough user instructions because these tests are performed at home without any professional supervision. In the current study, the researchers aimed at investigating the completeness and accuracy of information provided by different websites that sell home self-sampling and testing kits to detect SARS-CoV-2. They performed a website search on 23rd May 2020 using the Google search engine and screened 27 websites that sell a total of 41 kits (23 viral testing kits and 18 antibody testing kits) either in the UK or in the US. Websites that sell viral testing or antibody testing kits directly to users via direct purchase or insurance purchase were included in the study.
Study findings
For the analysis, the researchers extracted information from these websites between 23rd and 28th May 2020. The information extracted from the websites included test type and manufacturer details, timing of testing; test accuracy; advice provided regarding changing behavior considering test results; authorization; and test cost.
The researchers observed that about 78%, 24%, 29%, and 51% of testing kits were devoid of information about the manufacturer, test timing, test accuracy, and result interpretation, respectively. Regarding test accuracy, they found that about 66% of the kits are provided with specificity and sensitivity details as accuracy measures. Surprisingly, there were hardly any details about predictive values, which is defined as the probability of truly having a disease based on test results. Only 12% of the kits reported positive predictive values.
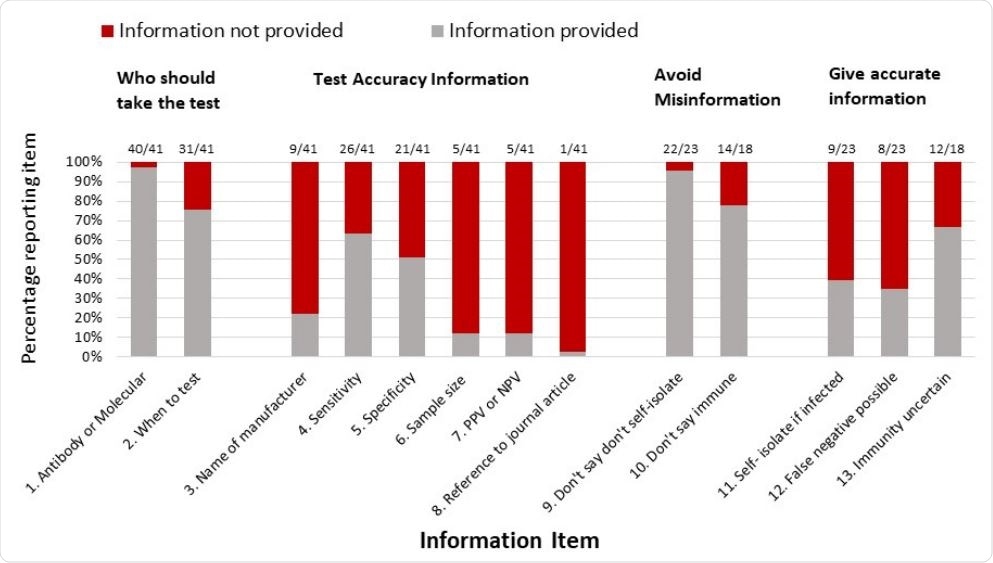
Proportion of home-sampling COVID-19 tests identified which met/did not meet each of the predefined criteria for clear communication to the consumer.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Regarding viral testing, it was mentioned in 39% of the kits that people who test positive should self-isolate, whereas, in 35% of the kits, it was mentioned that people with negative test results may still have the infection. Regarding antibody testing, 67% of the kits are provided with information that having a positive result does not ensure protection from future infection.
Regarding regulatory approval and endorsement, 41% of home-sampling antibody test kits available on the UK websites claimed to have a CE mark; However, in reality, there is currently no antibody test kit with regulatory approval for home sampling or testing. These inappropriately made claims were based on the approval for testing by experts using venous blood samples instead of finger-prick samples. Moreover, 24% and 29% of the UK websites selling viral testing kits claimed to have approvals from the regulatory bodies and policy-making bodies, respectively.
Study significance
The researchers believe that the observations made in the study will help understand the fact that inadequate or misleading information provided to users by test kit-selling websites can actually accelerate the pandemic rather than containing it through rapid home-based testing. People who perform home-based tests inappropriately or interpret test results inaccurately can be potential carriers of the virus, and thus, can increase the risk of disease transmission.
The researchers developed a guideline that mentions what should be included on the websites that sell home-based testing kits. According to them, a kit must be provided with information about the type of test, timing and procedure to perform the test, test accuracy, regulatory body approval status, test result interpretation, and implication of the test result.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources