Although two RNA-based severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine candidates elicited similar, dose-dependent neutralizing antibody titers in both younger and older adults, a recent study by researchers from the U.S. and Germany has shown that BNT162b2 is associated with less systemic reactogenicity in elderly individuals – supporting its selection for further phase 2/3 clinical trials. The study is currently available on medRxiv* preprint server.
.jpg)
Transmission electron micrograph of SARS-CoV-2 virus particles, isolated from a patient. Image captured and color-enhanced at the NIAID Integrated Research Facility (IRF) in Fort Detrick, Maryland. Credit: NIAID
The ongoing coronavirus disease (COVID-19) pandemic shows no signs of slowing down, causing more than 23.3 million infections and more than 806 thousand deaths as of August 24, 2020. Therefore, effective vaccines are urgently needed in order to halt this devastating public health crisis.
As a result, Pfizer and BioNTech launched, thus far, unprecedented and coordinated programs to compare four RNA-based COVID-19 pandemic vaccine candidates in umbrella-type clinical studies. The program was originated to select a single vaccine candidate and dose level for a critical global safety and efficacy trial.
Two lipid nanoparticle–formulated, nucleoside-modified RNA vaccine candidates were evaluated in more depth. One of these, known as BNT162b1, encodes the SARS-CoV-2 receptor-binding domain (RBD), while the other, BNT162b2, encodes the SARS-CoV-2 full-length spike protein to increase its potential for eliciting virus-neutralizing antibodies.
Previously reported data from vaccinating 18–55 year old adults with 10 μg or 30 μg of BNT162b1 hinted that it could indeed act as a promising COVID-19 vaccine candidate. And since more data in elderly individuals has been missing, a recent study filled this gap.
"Here we report the full set of safety and immunogenicity data from the Phase 1 portion of an ongoing randomized, placebo-controlled, observer-blinded dose-escalation U.S. trial that was used to select the final vaccine candidate, BNT162b2, as well as comparison of the safety and immunogenicity of both vaccine candidates", say researchers from notable institutions across the U.S. and Germany.
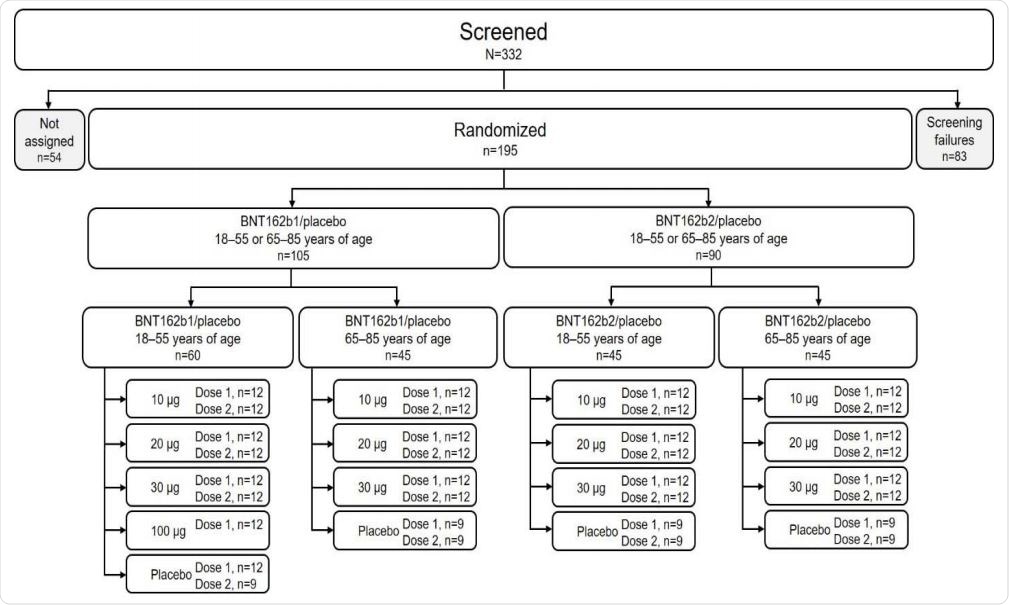
Disposition of participants. Participants not assigned were screened but not randomized because enrollment had closed.
Placebo-controlled, dose-escalation trial
In this study, safety and immunogenicity of BNT162b1 and BNT162b2 vaccines, when administered in varying dose levels, have been evaluated. More specifically, healthy adults aged 18–55 and 65–85 years were randomized in a placebo-controlled, observer-blinded dose-escalation trial. They received two doses at 21-day intervals of placebo or either of the two aforementioned vaccines.
Detailed safety appraisal included several endpoints (such as local reactions, systemic events, the use of pain medications or antipyretics, unsolicited or severe adverse events, laboratory abnormalities), and the researchers put protocol-specified safety stopping rules in place for all sentinel-cohort participants.
Immunogenicity was assessed with the use of SARS-CoV-2 serum neutralization assay and
RBD-binding or S1-binding IgG direct Luminex immunoassays before vaccination, at 7 and 21 days after the first dose, and 7 days (Day 21) and 14 days (Day 35) following the second vaccine dose.
Similar safety and immunogenicity profile
In a nutshell, the immune responses that were elicited by BNT162b1 and BNT162b2 were very similar. Dose-dependent SARS-CoV-2–neutralizing geometric mean titers for both vaccine candidates were comparable to or higher than the titers in a panel of SARS-CoV-2 convalescent sera.
As seen with other vaccines (and likely linked to immunosenescence), the immunogenicity of both vaccines decreased with age, generating lower humoral responses in 65–85-year-olds when compared to the 18–55 age group.
Nonetheless, BNT162b2 was associated with lower systemic reactogenicity (i.e., inflammatory response to vaccination such as injection-site pain and redness), particularly in elderly individuals included in this study.
Short-lived reduction in lymphocyte counts after vaccination, which was observed across age groups, happened without any evidence of associated clinical impact. This likely reflects temporary lymphocyte redistribution from the bloodstream to lymphoid tissues due to the immune stimulation.
Towards the large scale clinical trial
These results support the selection of the RNA-based COVID-19 BNT162b2 vaccine candidate for further large-scale safety and efficacy evaluations. The most important finding is that older BNT162b2 recipients disclosed no severe systemic events.
"The primary consideration driving this decision was the milder systemic reactogenicity profile of BNT162b2, particularly in older adults, in the context of comparable antibody responses elicited by both candidate vaccines", emphasize researchers in their medRxiv paper.
Of course, this interim report is not without certain limitations. First of all, data on immune responses or safety beyond 7 days after the second were not available at the time of publication. Furthermore, the relative importance of humoral and cellular immunity is not yet known, and there is always a sample size problem in these early-stage clinical trials.
"Many of the limitations to this study are now being addressed in the global Phase 2/3 portion of this study, while we expand our RNA vaccine manufacturing and distribution capacity", further say study authors.
The next steps will be to assess the safety and efficacy of two 30 µg doses of BNT162b2 in up to 30 thousand participants (randomized 1:1 with placebo) from diverse backgrounds – including individuals with occupational exposure, stable chronic underlying health conditions, as well as from various racial and ethnic backgrounds.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources