A team of researchers from the Netherlands demonstrated that prophylactic treatment with a high dose of human convalescent plasma or by using concentrated monoclonal antibodies could protect against disease following severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in an animal model. However, no protective effect was observed with a ten-fold lower dose of convalescent plasma. The results are currently available on the bioRxiv* preprint server.
Coronavirus disease (COVID-19), caused by SARS-CoV-2, is characterized by a wide array of symptoms such as fever, anosmia, dyspnea, cough, and muscle pains. In severe cases, infection is often complicated by acute respiratory distress syndrome (ARDS) that leads to respiratory insufficiency and, potentially, multi-organ failure.
Therefore, an effective treatment is of utmost importance, as the virus continues to circulate in many regions of the world (increasing the risk of future infection waves), and the quest for the effective and safe vaccine is still ongoing.
Several studies have already identified neutralizing antibodies against SARS-CoV-2 as a potential component of protective immunity. However, only a handful of studies to date have focused on evaluating the efficacy of convalescent plasma with antibodies to protect or prevent SARS-CoV-2 infection or COVID-19 in vivo.
Moreover, different human monoclonal antibodies against this putative agent have also been characterized for prophylactic and therapeutic use. But although efficient neutralization of SARS-CoV-2 has been proven in vitro, their efficacy has not been evaluated in living organisms.
Consequently, the researchers from Erasmus Medical Center and Utrecht University in the Netherlands used one specific monoclonal antibody and two doses of human convalescent plasma (differing ten-fold in the antibody concentration) in a hamster model to assess the efficacy of prophylactic antibody treatment in moderate to severe SARS-CoV-2 pneumonia.
Golden hamster as a golden model
In short, neutralizing antibodies were derived from six pooled convalescent plasma samples taken from PCR-confirmed COVID-19 patients. Additionally, a human monoclonal antibody 47D11 that targets a conserved epitope in the S1 viral domain has been used in a concentrated and purified form.
Since the Syrian golden hamster remains the only animal species thus far in which experimental SARS-CoV-2 infection gives rise to moderate or severe pneumonia – with specific clinical signs and viral shedding – it was justly selected as the test animal.
The enzyme-linked immunosorbent assay (ELISA) and plaque reduction neutralization test (PRNT) were used for serological appraisal purposes. Finally, samples from nasal turbinates and lungs of hamsters were collected for virus isolation and detection by real-time reverse transcription PCR, as well as for histological appraisal.
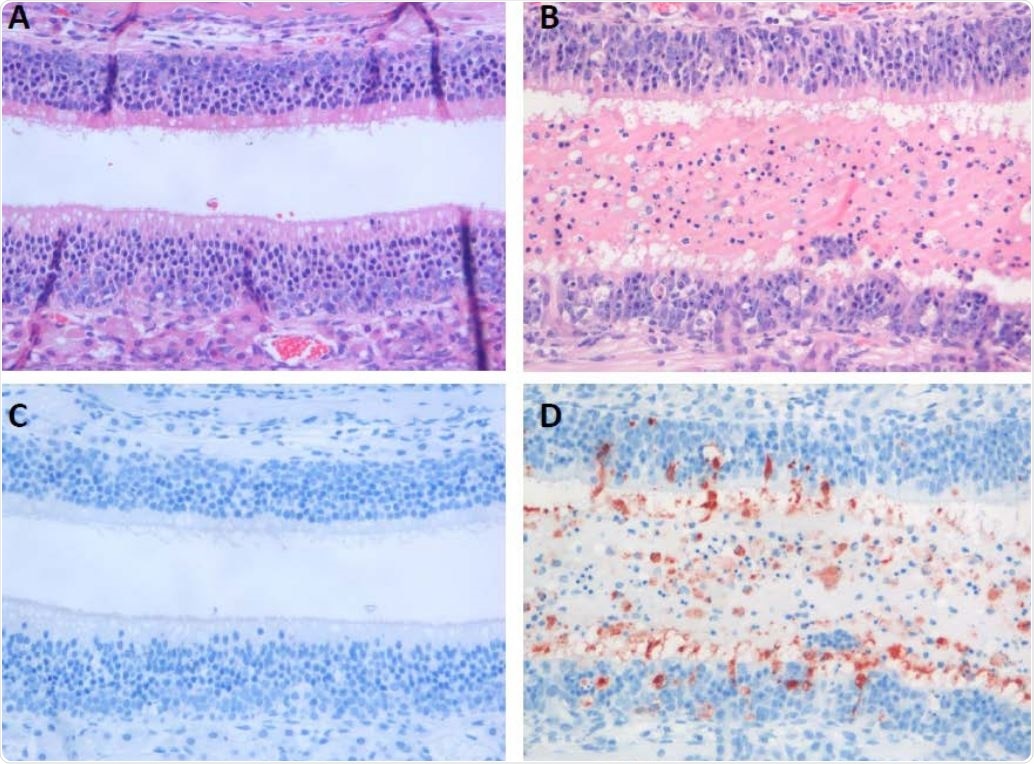
Histopathological changes and virus antigen expression in nasal turbinates of hamsters after challenge with SARS-CoV-2. In the nasal turbinate of a sham-inoculated hamster (left column), the nasal cavity is empty and the histology of the olfactory mucosa is normal (A). In a 511 serial section, there is no SARS-CoV-2 antigen expression (C). In the nasal turbinate of a non512 treated SARS-CoV-2-inoculated hamster (B and D), nasal cavity is filled with edema fluid mixed with inflammatory cells and debris and the olfactory mucosa is infiltrated by neutrophils (B). A serial section of this tissue shows SARS-CoV-2 antigen expression in many olfactory mucosal cells, as well as in cells in the lumen (C).
The promise of neutralizing antibodies
This study clearly shows that prophylactic treatment with neutralizing antibodies successfully prevents SARS-CoV-2 induced pneumonia in a hamster model. Furthermore, animals that were treated with a high dose of neutralizing antibodies did not lose weight or show any gross lesions in their lungs.
The critical finding was that the treatment resulted in a substantial reduction of viral replication and inflammation in the lungs. However, it has to be said that there was no protective efficacy with the use of convalescent plasma that has lower neutralizing antibody titers comparable to the median neutralizing titer in patients recovered from COVID-19.
"In addition, we show that while prophylactic treatment may prevent disease, animals still become infected and shed virus, indicating that transmission will not be blocked," caution study authors in this bioRxiv paper.
This underscores the importance of including parameters such as virus shedding, lung replication, as well as clinical and pathological disease determinants and adequately evaluating the efficacy of antibody treatment.
Monoclonal antibodies as a path forward
The study authors highlighted their main findings, "Our data show that prophylactic treatment with highly neutralizing monoclonal antibodies not only protects against weight loss and reduces virus replication in the lungs, it also limits histopathological changes in the lungs."
It is clear that treatment using convalescent plasma provides only partial protection, and only in instances when plasma with high neutralizing titers is used. Therefore, if the decision is made to use convalescent plasma, only donors with high levels of neutralizing antibodies should be considered.
Nonetheless, given the highly variable antibody response in patients, the pool of suitable donors for immunoglobulin therapy is severely limited. This can be circumvented with the usage of in vitro produced monoclonal antibodies, and the results of this study suggest this may indeed be the more favorable route for developing an effective treatment option.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources