Charles Murry and colleagues found that the virus directly infected cardiomyocytes, impaired their electrophysiological and contractile properties, and eventually caused cell death.
"These results support the hypothesis that independent of inflammation or coagulopathy, SARS-CoV-2 can cause direct functional heart damage," writes the team.
The researchers say that as well as efforts to control systemic inflammation in patients with coronavirus disease 2019 (COVID-19), the use of antiviral or cardioactive drugs should also be considered to help prevent long-term cardiovascular complications.
A pre-print version of the paper is available on the bioRxiv* server, while the article undergoes peer review.
Potential direct and indirect effects of SARS-CoV-2 infection
Since the first cases of COVID-19 were first identified in Wuhan, China, late last year, the rapid spread of SARS-CoV-2 has led to a pandemic where more than 25 million people globally have now been infected, and more than 848,000 have died.
Although COVID-19 is primarily regarded as a respiratory illness, it can involve the cardiovascular system and worsen pre-existing problems, as well as causing new ones to develop.
The adverse cardiovascular effects are significantly contributing to increased mortality among COVID-19 patients, yet so far, the underlying mechanisms of cardiac involvement are not yet clear, say Murry and team.
Following infection with SARS-CoV-2, the immune system can launch an excessive uncontrolled release of inflammatory cytokines that can exacerbate existing cardiovascular problems and even cause heart failure. Furthermore, COVID-19 is associated with the development of coagulopathies that can also damage the heart.
On the other hand, SARS-CoV-2 might directly cause heart damage after entering cardiomyocytes via binding of the viral surface "spike" protein to the host cell receptor angiotensin, converting enzyme 2 (ACE2).
The authors say a growing number of reports have shown the presence of the SARS-CoV-2 genome in heart tissue and evidence of viral myocarditis among patients with COVID-19, including people with asymptomatic illness.
This suggests that "SARS-CoV-2 could exhibit cardiac tropism and thus directly impair cardiac function," they write. "Myocardial infarction, arrhythmias, and heart failure are the most common cardiovascular complications observed in COVID-19 patients."
What did the current study involve?
Now, the team has used human pluripotent stem cell-derived cardiomyocytes (hPSC-CMs) and three-dimensional engineered human heart tissues (3D-EHTs) to investigate the cardiac tropism of SARS-CoV-2 and study infected cardiomyocytes to clarify the functional changes that might underly cardiac symptoms in cases of COVID-19.
The researchers found that the ACE2 protein was strongly expressed in hPSC-CMs derived from multiple lines, including RUES2 female embryonic stem cells (hESCs), H7 female hESCs, and WTC11c-induced male pluripotent stem cells (hiPSCs),
Collectively, the team concluded that hPSC-CMs express proteins that make them susceptible to SARS-CoV-2 infection.
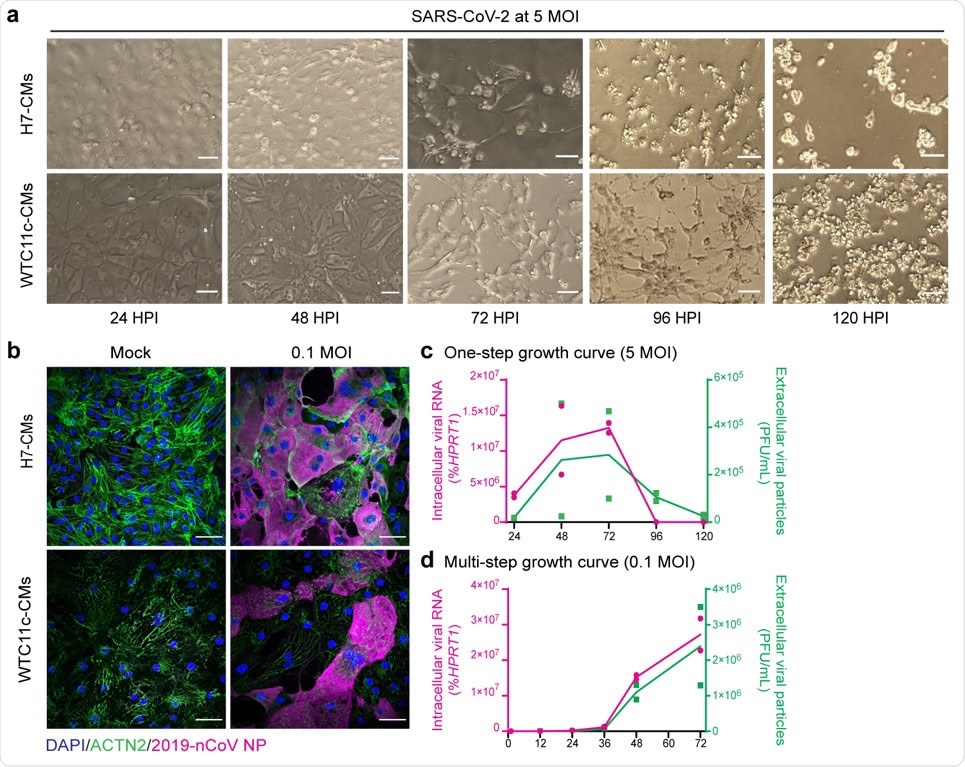
hPSC-CMs are permissive to SARS-CoV-2 infection and replication. (a) Cytopathic effects of SARS-CoV-2 at 5 MOI in H7 hESC-CMs and WTC11c hiPSC-CMs during a time course of 120 h. Scale bars: 100 μm. (b) Immunofluorescent staining of H7 hESC-CMs and WTC11c hiPSC-CMs at 48 HPI with SARS-CoV-2 dose of 0.1 MOI. Scale bars: 50 μm. Individual channels are shown in Supplementary Fig. 2c. (c) One-step viral growth curve in H7 hESC-CMs infected with SARS-CoV-2 at 5 MOI over a time course of 120 h. (d) Multi-step viral growth curve in H7 hESC-CMs infected with SARS-CoV-2 at 0.1 MOI over a time course of 72 h. For both c and d, lines connect the means of two independent experiments. Viral RNA indicating intracellular viral replication is plotted on the left y axis as % of HPRT1. Viral particles secreted in the supernatant are plotted on the right y axis as PFU/mL.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Testing the cells' susceptibility to SARS-CoV-2 infection
Since ACE2 expression was highest among the H7 and WTC11c-derived hPSC-CMs, the researchers tested functional susceptibility to infection in these cell lines.
They incubated highly-pure hPSC-CMs with the SARS-CoV-2/Wa-1 strain at a multiplicity of infection (MOI; the number of viable virions per cell) of either 0.1 or 5.
The team observed significant, disseminated cytopathic changes in both H7 hESC-CMs and WTC11c hiPSC-CMs, that were accelerated at 5 MOI.
Most importantly, cells stopped beating and started to die as early as 48-hours post-infection in both cell lines.
"Remarkably, even in the absence of major cytopathic effects, SARS-CoV-2 infection rapidly resulted in reduced beating rate, lower depolarization spike amplitude, and decreased electrical conduction velocity," write the researchers.
"These properties of SARS-CoV-2 infection in cardiomyocytes could explain the high rate of arrhythmia (~14%) which has been observed in COVID-19 patients," they add.
Immunofluorescence staining revealed the substantial presence of viral factors in the cytoplasm of both cell lines, thereby confirming that SARS-CoV-2 had directly infected hPSC-CMs.
Assessing the effects in engineered human heart tissue
On evaluating the contractile properties of infected hPSC-CMs using 3D-EHTs, the team found that contractile behavior was significantly impaired.
The authors say this shows that the mechanical function of cardiomyocytes is impacted by SARS-CoV-2 infection and that this could contribute to whole-organ cardiac dysfunction in patients.
"These results support the hypothesis that, independent of inflammation or coagulopathy, SARS-CoV-2 can cause direct functional heart damage," write the researchers.
"COVID-19 patients are commonly treated with steroids to control systemic inflammation. However, our data suggests that treatments aimed to control the direct damage of SARS-CoV-2, e.g. antiviral medications and/or cardioactive drugs, should also be taken into consideration to prevent long-term cardiovascular complications," concludes the team.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Murry C, et al. SARS-CoV-2 infects human pluripotent stem cell-derived cardiomyocytes, impairing electrical and mechanical function. bioRxiv 2020. doi: https://doi.org/10.1101/2020.08.30.274464
- Peer reviewed and published scientific report.
Marchiano, Silvia, Tien-Ying Hsiang, Akshita Khanna, Ty Higashi, Leanne S. Whitmore, Johannes Bargehr, Hongorzul Davaapil, et al. 2021. “SARS-CoV-2 Infects Human Pluripotent Stem Cell-Derived Cardiomyocytes, Impairing Electrical and Mechanical Function.” Stem Cell Reports 16 (3): 478–92. https://doi.org/10.1016/j.stemcr.2021.02.008. https://www.cell.com/stem-cell-reports/fulltext/S2213-6711(21)00088-6.