In the ongoing COVID-19 pandemic, diabetes has been unequivocally established to be a risk factor for mortality and severe disease. Moreover, several new clusters of new-onset diabetes have been identified, as well as the worsening of diabetic conditions, due to the occurrence of diabetic ketoacidosis and hyperosmolar hyperglycemia.
This has led to the proposition that SARS-CoV-2 is a diabetogenic virus, by causing toxicity to the beta-cells of the pancreatic islets that secrete insulin. In vitro studies demonstrate that the entry of the virus into a human host cell is dependent on the interaction of the viral spike protein with the angiotensin-converting enzyme (ACE) 2 on the cell. This is followed by the proteolytic cleavage of the spike protein, by the serine protease TMPRSS2.
SARS-CoV Causes Acute Diabetes?
An earlier pathogenic coronavirus, SARS-CoV, which caused a more limited outbreak between 2002 and 2004, also uses the ACE2 receptor. Prior research on autopsy samples from patients who died of this infection reported that ACE2 was found on pancreatic islet cells from one donor (though the type of cell was not clear). Thus, they first floated the idea that virus-ACE2 engagement causes islet damage and triggers acute diabetes. This was reversible once the infection was cleared.
Do Beta Cells Express ACE2?
The same authors reported recently that they induced beta-like cells from human pluripotent stem cells (hPSCs). Tests on these cells, as well as the beta cells from human pancreatic islets isolated from the organ, were found to express ACE2, supporting their hypothesis of direct viral injury to these cells.
Looking for ACE2/TMPRSS2 Expression in Beta Cells
The current study aims to carry out a more in-depth analysis of pancreatic cells for the expression of both ACE2 and TMPRSS2 from individuals with and without diabetes. The researchers found no evidence of either of these in human islet endocrine cells in diabetic or non-diabetic donors. This means they say that a direct effect of the virus on the induction of diabetes is unlikely.
In fact, the expression of mRNA transcribing either ACE2 and TMPRSS2 is minimal in human alpha and beta cells.
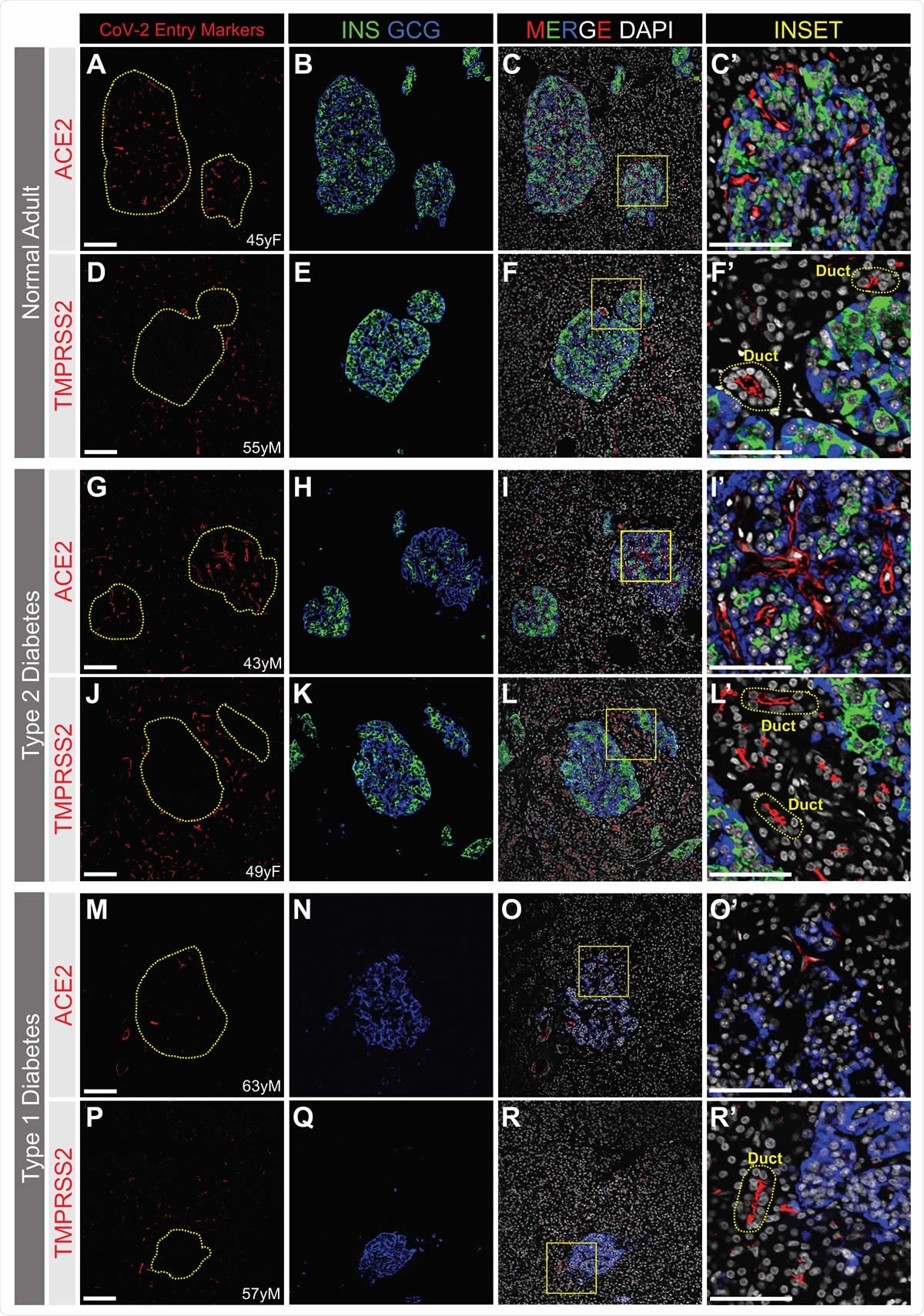
ACE2 and TMPRSS2 Protein is Not Detected by Immunofluorescence in a or b Cells from Normal, T2D or T1D adult donors. SARS-CoV-2 cell entry markers ACE2 (antibody ab15348) and TMPRSS2, both shown in red, are not detected in islet a cells (GCG, blue) or b cells (INS, green) in pancreatic sections from adult donors without diabetes (A-H) or donors with type 2 (I-N) or type 1 (O-V) diabetes. Insets are depicted by a yellow box. DAPI (white). Scale bars are 100 μm (A-V) and 25 μm (Insets). Human islet and pancreatic donor information is available in Table S1 (A-D, donors N3, N7, N9, N8; E-H, donors N14, N12, N11, N10; I-L, donors 2L, 2B, 2G, 2I; M-N, donors 2H, 2G; O-R, donors 1B, 1D, 1C, 1A; S-V, donors 1H, 1K, 1J, 1G).

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
RNA Sequencing Fails to Find ACE2/TMPRSS2
The researchers first used data from two bulk RNA-sequencing (RNA-seq) datasets, comparing the mRNA expression for these two enzymes in alpha and beta cells from human pancreatic islet tissue with that of important genes encoding, for instance, transcription factors, in islet cells. These genes are typically expressed at low levels in these cells.
However, the median level of ACE2 and TMPRSS2 mRNA expression was significantly lower than the mRNA for these islet-enriched genes. That is, these two mRNAs were present at 84% and 92% lower expression levels compared to those of some DNA-binding transcription factors within the alpha and beta islet cells.
They also found that single-cell (sc) RNA-seq analysis of human pancreatic islets demonstrated the absence or minimal expression of mRNAs transcribing the ACE2 and TMPRSS2 genes. The results they used include those obtained from over 25,000 cells of the Human Pancreas Analysis Program (HPAP) database donated by 11 healthy donors, as well as three smaller datasets. Yet, they found ACE2/TMPRSS2 expression in less than 1% of beta cells across all datasets.
Low ACE2/TMPRSS2 Co-Expression in Non-Islet Cells
The exocrine cells showed ACE2 positivity at moderate to high levels for 5% of endothelial cells and pericytes, in the HPAP dataset, but only up to 3% in the other three. This difference is not significant and is most likely to be due to varying numbers of cells in each analysis, by up to 20 times more in the HPAP database compared to the smallest set. A pooled analysis shows that overall, less than 1% of acinar or ductal cells had ACE2 expression, but 35% of both kinds of cells expressed TMPRSS2. In contrast, in the smallest study, ACE2 and TMPRSS2 expression in these cells were at the level of 20% and over 75%, respectively.
Since these are key enzymes for SARS-CoV-2 infection, the researchers found that both were expressed together in less than 1% of four cell types (acinar, ductal, endothelial, and stellate) in three datasets. In the fourth, the percentage of co-expression was 5% and 15% for acinar and ductal cells, respectively.
The researchers point out, “Notably, no islet endocrine cells co-expressed ACE2 and TMPRSS2 in any of these four datasets.”
Direct Visualization Fails to Detect ACE2
Secondly, the researchers moved from transcriptomics to direct visualization. They used immunostaining on islet cryosections with the same ACE2 antibody reported by earlier investigators. However, they failed to detect ACE2 on beta cells, but only on the microvasculature.
The researchers point out that the difference might possibly be due to the use of stem cell-derived beta cells, which are considered to be juvenile-like cells rather than functionally mature, or the immortalized beta cells in culture, in an earlier study. To rule out this possibility, they then repeated the process using sections from pancreatic tissue obtained from children aged 5 years or less (one was only 5 days old). However, they still did not find ACE2.
ACE2 Localized to Microvasculature
Next, the investigators looked at donor pancreatic tissue from diabetic and non-diabetic donors. The diabetic donors had either type 1 or type 2 diabetes. Here again, ACE2 was not found on either alpha or beta cells, but the microvascular structures in both the healthy donors and those with type 2 diabetes.
They repeated their analysis with three other ACE2 antibodies used by several other researchers, but failed to find this protein on islet cells.
TMPRSS2 Localized to Ducts
They also did not find TMPRSS2 within islets in healthy or type 1 diabetic donors, but it was localized on exocrine tissue, on duct epithelium. Overall, they concluded that these proteins are not found on islet endocrine cells from either healthy or diabetic donors. The scientists now think that the earlier findings may be due to key differences in the way the experiment was set up and perhaps artifacts.
DPP4 Found on Alpha Cells
Again, following a more recent in silico study that indicated the possibility of viral binding to human dipeptidyl peptidase 4 (DPP4) in enabling viral entry, they searched for this protein on the human pancreas. They found that it was localized to alpha but not beta cells of all donors, diabetic or not.
This finding agrees with the transcriptomic analysis based on all four datasets, which also demonstrated that these cells were DPP4-enriched. However, the absence of TMPRSS2 indicates, they say, that DPP4 is probably not related to viral cell entry into beta cells.
ACE2 is Localized to Capillaries of Islets and Exocrine Tissue
The researchers then looked into the staining pattern of ACE2 in the non-endocrine cells of the pancreas, specifically to find out if it was localized in the microvasculature. They concluded that this was the case, with the perivascular capillaries of the islets as well as exocrine tissue showing ACE2 expression.
They suggest that this protein is expressed on pericytes, the cells that envelope capillary endothelial cells. However, since there is no TMPRSS2, this cell would not be targeted by SARS-CoV-2. And in fact, though both these proteins are found on duct epithelium, they are not found together.
TMPRSS2 is on the apical surface of duct cells in the exocrine pancreas, but not ACE2. In the few instances where they are both found together, they are separately localized. The researchers say they may represent a possible viral target.
In type 1 diabetes, ACE2 expression is low compared to healthy donors.
Implications
Most researchers agree that ACE2 and TMPRSS2 are probably the cellular machineries required for viral entry into host cells, though other routes are theoretically possible. In this scenario, the current findings do not show a permissive role for pancreatic beta cells.
Some limitations of the current study include the fact that the researchers relied on the demonstration of these proteins and not on looking at viral binding or entry into the beta cells. Finally, they did not measure the expression of these proteins in diabetic patients with COVID-19, so that they cannot rule out changes in these receptors in such patients.
The study sums up, “Altogether, these findings greatly reduce the likelihood that SARS-CoV2 can bind and enter human b cells and have direct cytotoxicity.” Instead, they favor the explanation that glucose homeostasis is probably impacted by inflammation or damage to liver, muscle, or fat cells, which needs to be borne out by further research.
Source

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources