Researchers in the UK and Taiwan have demonstrated the potential of a new candidate vaccine against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – the virus responsible for the current coronavirus disease 2019 (COVID-19) pandemic.
Low doses of the vaccine triggered a potent neutralizing antibody response in mice and pigs that was stronger than the response induced by serum taken from patients who had recovered from COVID-19.
The virus-like particle (VLP) vaccine displays the receptor-binding domain (RBD) of the viral “spike” protein – the surface structure that SARS-CoV-2 uses to bind to and access host cells.
Alain Townsend (University of Oxford) and colleagues, who used technology called SpyTag/SpyCatcher to assemble the RBD of SARS-CoV-2 onto a VLP, have called the resulting vaccine candidate RBD-SpyVLP.
The team says the potent and polyclonal antibody response the vaccine-induced highlights its potential as an effective and affordable solution to addressing the current clinical and logistic challenges faced in the fight against COVID-19.
A pre-print version of the paper I available on the server bioRxiv*, while the article undergoes peer review.
.jpg)
Colorized scanning electron micrograph of a cell (blue) heavily infected with SARS-CoV-2 virus particles (red), isolated from a patient sample. Image captured at the NIAID Integrated Research Facility (IRF) in Fort Detrick, Maryland. Credit: NIAID

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
No effective vaccines are currently available
Following the first cases of COVID-19 in Wuhan, China, late last year, SARS-CoV-2 swept the globe and was declared a pandemic by the World Health Organization on March 11th this year. Now, the virus has infected more than 25.59 million people worldwide and claimed the lives of more than 852,000.
Currently, no effective vaccines are available, although about 25 are undergoing clinical testing, and around 140 are in the preclinical stages of evaluation. These vaccine candidates are based on the viral vector, viral protein subunits, viral DNA and RNA, and VLPs, with most of them focused on the immunogenic potential of the SAR-CoV-2 spike protein.
“Protein subunit vaccines generally have good safety profiles”
SARS-CoV-2 binds the human angiotensin-converting enzyme 2 (ACE2) receptor using the RBD of the Spike S1 subunit, which enables the virus to enter host cells.
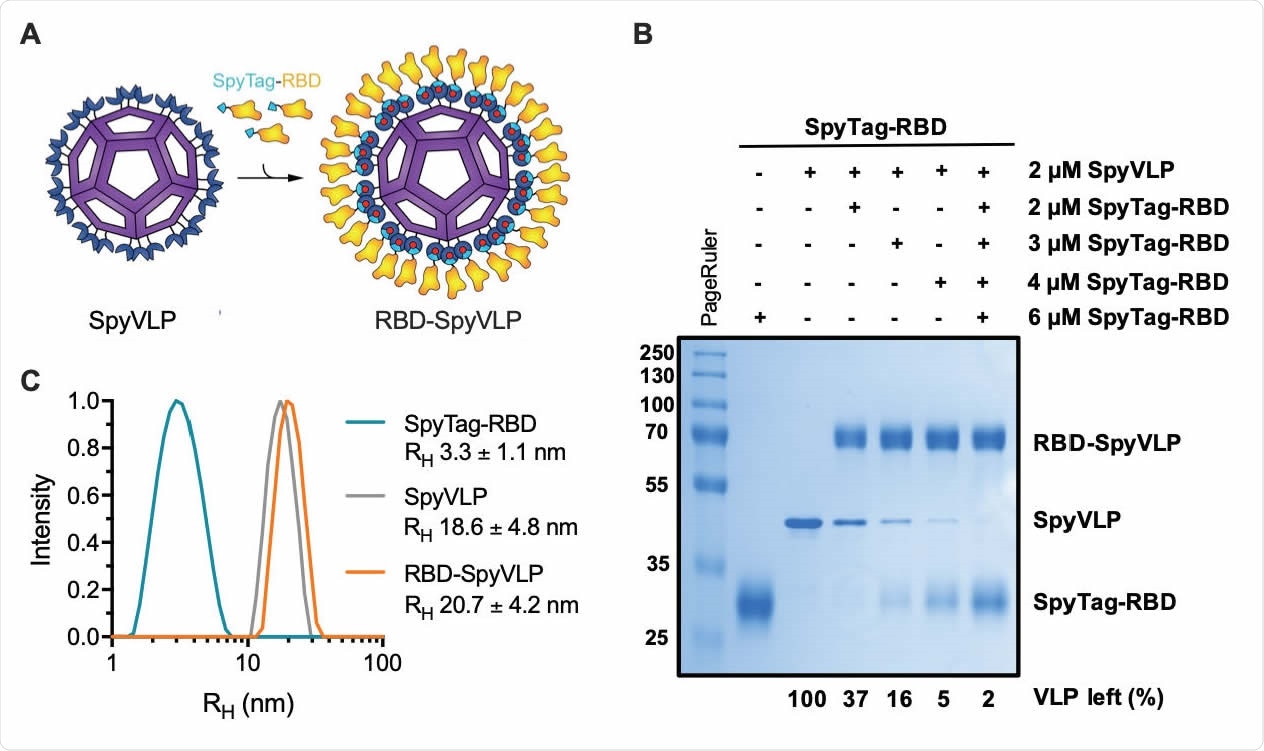
SpyTag-RBD can be efficiently conjugated to SpyCatcher003-mi3 VLP. (A) Schematic diagram of the RBD-SpyVLP vaccine candidate, consisting of SpyCatcher003-VLP conjugated with SpyTag-RBD. The isopeptide bonds formed spontaneously between SpyTag and SpyCatcher are indicated with red dots. (B) Conjugation of SpyCatcher003-mi3 with SpyTag-RBD at various ratios. Reactions were performed at 4 °C overnight and analysed using SDS-PAGE with Coomassie staining and densitometry, with the percentage of unreacted VLP shown. (C) Dynamic light scattering (DLS) characterisation of SpyTag-RBD, SpyVLP, and conjugated RBD-SpyVLP (n=3, values shown as mean±SD). RH= hydrodynamic radius.
“Of the many vaccine platforms, protein subunit vaccines generally have good safety profiles, and their production is rapid and easily scalable,” writes Townsend and colleagues.
The researchers say numerous studies have recently demonstrated that the RBD of the SARS-CoV-2 spike protein triggers the production of neutralizing antibodies. Some studies have also shown that most of the potent neutralizing antibodies isolated from SARS-CoV-2- infected patients bind to the RBD.
“We, therefore, chose to study the immunogenicity of RBD,” says the team.
What did the current study involve?
To improve immunogenicity, the team used SpyTag/SpyCatcher technology to conjugate SARS-CoV-2 RBD onto a VLP called mi3.
The use of VLPs to display protein antigens has previously been shown to increase immunogenicity by enabling drainage to lymph nodes and enhancing uptake by antigen-presenting cells.
Using a panel of monoclonal antibodies isolated from convalescent patients, the team showed that all of the epitopes that could potentially trigger the generation of protective RBD-specific antibodies are present in RBD-SpyVLP.
“Since RBD-SpyVLPs induce antibody responses that target multiple epitopes on the RBD, the chance of selecting neutralization-escape mutants should be greatly reduced,” said Townsend and colleagues. “Circulating SARS-CoV-2 stains are constantly mutating, and the likelihood of persistence of the virus in the human population is high.”
What happened when mice and pigs were vaccinated?
Only negligible antibody responses were seen when mice were vaccinated with 0.1 µg or 0.5 µg doses of RBD alone, but strong responses were seen once the RBD was displayed on the VLP.
Serum from mice that received either 0.1 µg or 0.5 µg doses of RBD-SpyVLP exhibited high levels of antibody against the RBD of SARS-CoV-2, as well as the full-length spike protein and showed potent ACE2 blocking activity.
“All of these responses were higher than the levels found in plasma from convalescent humans,” write the researchers. “These results confirm the enhanced immunogenicity of RBD when displayed on SpyVLPs.”
RBD-SpyVLP vaccination also induced high titers of neutralizing antibodies in pigs using a dose that the authors intended to test in human trials (5 µg). The team reports that at a dose of 5 µg, similar neutralization titers were observed as when 100 µg of spike protein was administered.
The researchers say the finding that the RBD-SpyVLP vaccine candidate is highly immunogenic in mice and pigs suggests that it could potentially elicit protective antibody responses against SARS-CoV-2 in humans.
The vaccine was also resilient
Furthermore, when the team tested the resilience of RBD-SpyVLP, they found it was stable at ambient temperature, resistant to freeze-thaw, and could be lyophilized (freeze-dried) and reconstituted, without any significant loss in activity or immunogenicity.
“This resilience may not only simplify vaccine distribution worldwide, especially to countries where cold-chain [low-temperature storage] resources are incomplete but also reduce the overall vaccine cost by removing cold chain dependence,” says Townsend and colleagues.
The researchers conclude that overall, the findings show that the RBD-SpyVLP is a potent and adaptable vaccine candidate that could potentially help to address the clinical and logistic challenges faced in combating the COVID-19 pandemic.
“We are currently investigating cheaper and more scalable alternatives to produce RBD-SpyVLP to cope with the global demand for a SARS-CoV-2 vaccine,” they add.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Townsend A, et al. A COVID-19 vaccine candidate using SpyCatcher multimerization of the SARS-CoV-2 spike protein receptor-binding domain induces potent neutralising antibody responses. bioRxiv, 2020. doi: https://doi.org/10.1101/2020.08.31.275701
- Peer reviewed and published scientific report.
Tan, Tiong Kit, Pramila Rijal, Rolle Rahikainen, Anthony H. Keeble, Lisa Schimanski, Saira Hussain, Ruth Harvey, et al. 2021. “A COVID-19 Vaccine Candidate Using SpyCatcher Multimerization of the SARS-CoV-2 Spike Protein Receptor-Binding Domain Induces Potent Neutralising Antibody Responses.” Nature Communications 12 (1): 542. https://doi.org/10.1038/s41467-020-20654-7. https://www.nature.com/articles/s41467-020-20654-7.