We were initially studying pathways/mechanisms that regulate the clearance of extracellular cargoes, such as dying cells, and the impact these pathways have on immune system activation. We saw Alzheimer’s Disease (AD) as an ideal model to study these pathways in a disease-relevant setting.
It has been well established that AD is characterized by beta-amyloid accumulation, peptides which are known to be internalized and cleared by resident brain immune cells called microglia, and by high levels of neuroinflammation stemming from microglial activation.
Image Credit: Atthapon Raksthaput/Shutterstock.com
What is the LC3-associated endocytosis (LANDO) pathway and how did you first discover this?
We identified LANDO as a unique cellular pathway while investigating similar mechanisms including LC3-associated phagocytosis (LAP) more conventional cellular recycling pathways including autophagy. We found that components of the autophagy pathway function to conjugate a small microtubule-associated protein called LC3 to endosomes containing beta-amyloid in microglia.
Calling this pathway LANDO, we further found that LANDO is of critical importance to the recycling of receptors on microglia that recognize beta-amyloid and facilitate its internalization and ultimately clearance by microglia.
Can you describe your research that led to the discovery that LANDO protects against neuroinflammation?
While studying mice that were deficient in LANDO that were on a background that generates AD (5xFAD mouse) we found that LANDO-deficiency led to robust increases in not only beta-amyloid deposition in the brain but pervasive neuroinflammation.
As we further investigated isolated microglia, it was clear that cells that lacked LANDO were hypersensitive to beta-amyloid and increased levels of inflammatory mediators.
The direct mechanism that is linking LANDO to the suppression of inflammation remains elusive, however, we are rigorously investigating this facet to better understand the underlying biology.
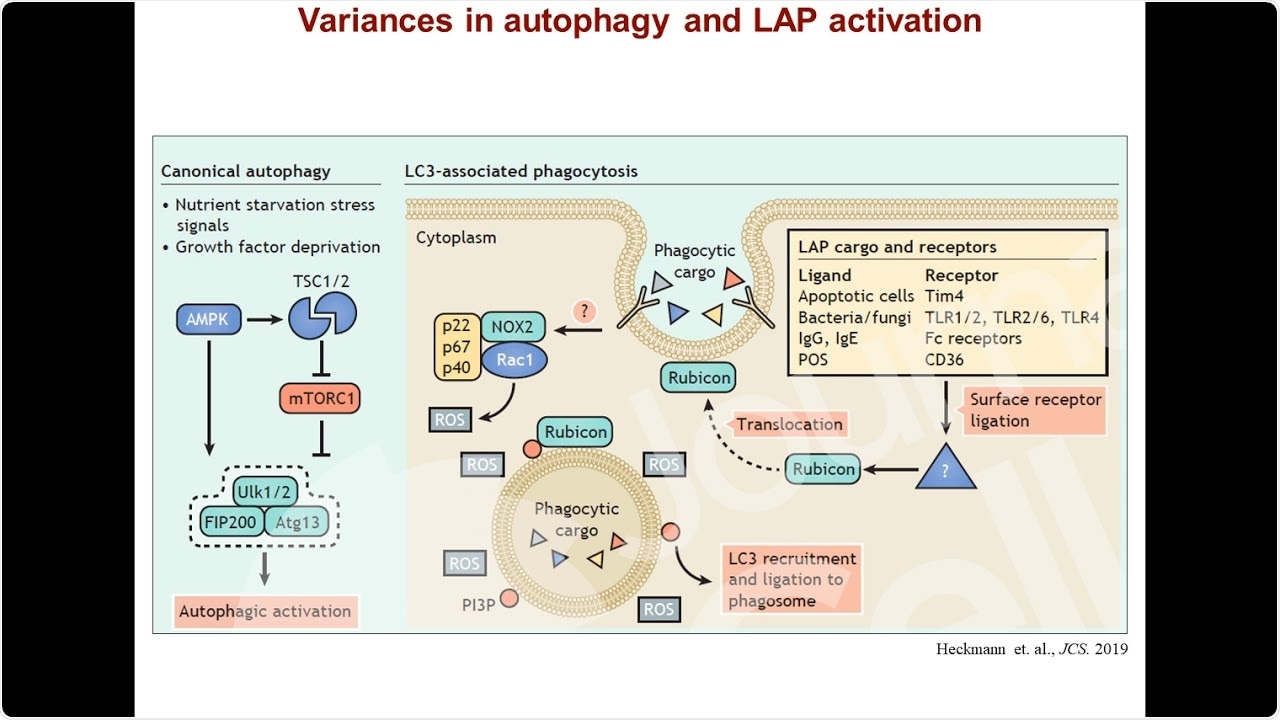
Image Credit: https://www.youtube.com/watch?v=QTvH9g2UDRw
In your research, you also identified a novel function of protein ATG16L. What is the role of this protein in LANDO?
ATG16L is a critical component of a complex in the autophagy pathway that regulates the processing of this protein LC3 leading to its conjugation on various vesicle structures, including endosomes in LANDO. Deletion of ATG16L would result in the abrogation of not only LANDO but other pathways including autophagy and LAP.
However, the particular mutant used in the present study, the WD-domain deletion mutant, fails to impair autophagy but does result in the loss of LANDO, thereby genetically discriminating the two pathways.
You carried out your research in both a mouse model and on human tissue samples. Can you describe the results of this research?
In the mouse model that was deficient in LANDO, we found they presented with a spontaneous AD-like pathology that replicated most clinical features of human disease as they age. These included not only beta-amyloid and tau pathology, but also consistent neuroinflammation, neurodegeneration, neuronal dysfunction, and resulting behavior and memory impairment.
Moreover, we found that the expression of components that regulate LANDO, including our previously established protein Rubicon as well as ATG16L, were significantly decreased in the brains from human AD patients compared to their age-matched healthy counterparts.
While these data do not provide us direct evidence that deficiency in LANDO is driving or contributing to human AD, they do provide a strong correlation that pathways that utilize these proteins (such as LANDO) are likely being affected in human disease.
How could your research help to identify a potential new treatment for Alzheimer’s?
In our present study, we found that therapeutically targeting neuroinflammation was advantageous and resulted in a reduction in active neurodegeneration, tau pathology, and led to improved behavior and memory in our mouse model.
These results suggest that neuronal loss is not necessarily a parameter that cannot be either overcome or circumvented when treating neurodegenerative diseases such as AD, as treated mice had very robust improvements in memory. Cumulatively, our data supports targeting neuroinflammation and/or LANDO as a viable therapeutic avenue in AD.
What further research is needed to be carried out before a treatment could be proposed?
With respect to targeting neuroinflammation directly, the particular compound we used is not clinically approved due to the potential for systemic side effects in humans. However, similar compounds that target the same inflammation producing complex in AD that the compound we used, are currently in clinical trials, so there is quite a bit of promise that those therapies will come to fruition.
Since LANDO is such a newly identified pathway, we are still learning about the basic biology and regulation of the pathway in microglia. Therefore, while targeting LANDO as therapy is certainly tempting to speculate upon, more investigation is necessary to elucidate a possible avenue for exploiting it therapeutically.

What are the next steps in your research into Alzheimer’s Disease?
While it is clear that neuroinflammation is a major component of AD, our full understanding of how inflammatory processes are regulated, and the consequences of inflammation are still in their infancy. As a starting point, we aim to further our understanding of how LANDO and inflammation actually intersect at a mechanistic level.
Additionally, we would like to further our knowledge of how, when, and where inflammatory mediators are functioning in the AD brain using novel models to profile immune architecture in the brain, what we like to call “neuroimmunomics”.
Where can readers find more information?
About Dr. Bradlee L. Heckmann
Brad is currently transitioning to his recent faculty appointment as a neuroimmunologist at the Byrd Alzheimer’s Institute and Assistant Professor in Molecular Medicine at the University of South Florida Morsani College of Medicine. 
For the duration of the present work, he has held the John H. Sununu Endowed Fellowship in Immunology at St. Jude Children’s Research Hospital, working alongside Dr. Douglas Green.
Throughout his career, his research has focused on the regulation of the autophagy pathway to modulation of lipid homeostasis with his most recent work investigating the noncanonical use of the autophagy proteins in regulating immune function in the brain.
This work has culminated with the discovery of LC3-associated endocytosis and its putative role in AD alongside and under the mentorship of Dr. Douglas Green.