The COVID-19 pandemic has now entered its eighth month, but severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection continues to spread in many parts of the world. Researchers are still searching for effective pharmaceutical interventions. A recent study published in the preprint server medRxiv* in September 2020 shows that the already approved drug families called P13K/mTOR and topoisomerase inhibitors may be useful in reducing individual susceptibility to the virus.
SARS-CoV-2 virus binding to ACE2 receptors on a human cell, the initial stage of COVID-19 infection. Illustration Credit: Kateryna Kon / Shutterstock
SARS-CoV-2 is a single-stranded RNA virus that uses spike glycoproteins to attach to the host cell and achieve host cell entry. This engagement is via the host cell receptor, angiotensin-converting enzyme 2 (ACE2), which is abundantly expressed on airway epithelial cells. Once the virus attaches to the receptor, the spike protein is cleaved by the transmembrane protease serine 2 (TMPRSS2). This leads to the internalization of the virus, where it is then able to fuse with the cell membrane and enter the cytosol.
The ACE2 gene is thus the primary target for the prevention of SARS-CoV-2 infection. Prior research shows that its expression on a set of cell lines is linked to the in vitro susceptibility of the given cell to the virus. Also, since the enzymes in this viral attachment and entry are not known to provide any vital function in the human host, it should be easy to target them without cytotoxic host effects.
Testing Drugs for Reduced ACE2 Expression
The researchers used a drug library, namely, the Library of Integrated Network-Based Cellular Signatures (LINCS) program, an open resource containing the expression profiles of cells exposed to several agents that disrupt normal cell activity. They evaluated the gene expression patterns found in association with different drug exposures in LINCS for over 1,800 drugs for which the cell interactions were available at varying doses, from 0.01 uM to 10 uM, across seven cell lines.
They were able to identify two major drug classes, which showed the highest reduction in ACE2 expression, namely, topoisomerase inhibitors and PI3K/mTOR pathway inhibitors. The former was found to be associated with the most significant fall in ACE2 expression, and often this was proportional to the dose, as was also the case with PI3K/mTOR inhibitors. The former includes drugs like camptothecin, SN-38, and Genz-644282, while the latter, including PF- 04691502, GDC-0980(RG7422), and Taselisib, also showed reduced ACE2 expression, in direct correlation to the dose. Two AKT inhibitors, which also are involved in the PI3K/mTOR pathway, Afuresertib, and MK-2206, also cause lower ACE2 expression.
They also looked at whether cancer patients who were treated with drugs that reduced ACE2 expression had lower odds of testing positive for the virus compared to cancer patients on other drugs. In this retrospective clinical review, they found that among 535 patients on these ACE2-suppressing drugs, the odds of testing positive for the virus were 35% lower than with other anti-neoplastic drugs.
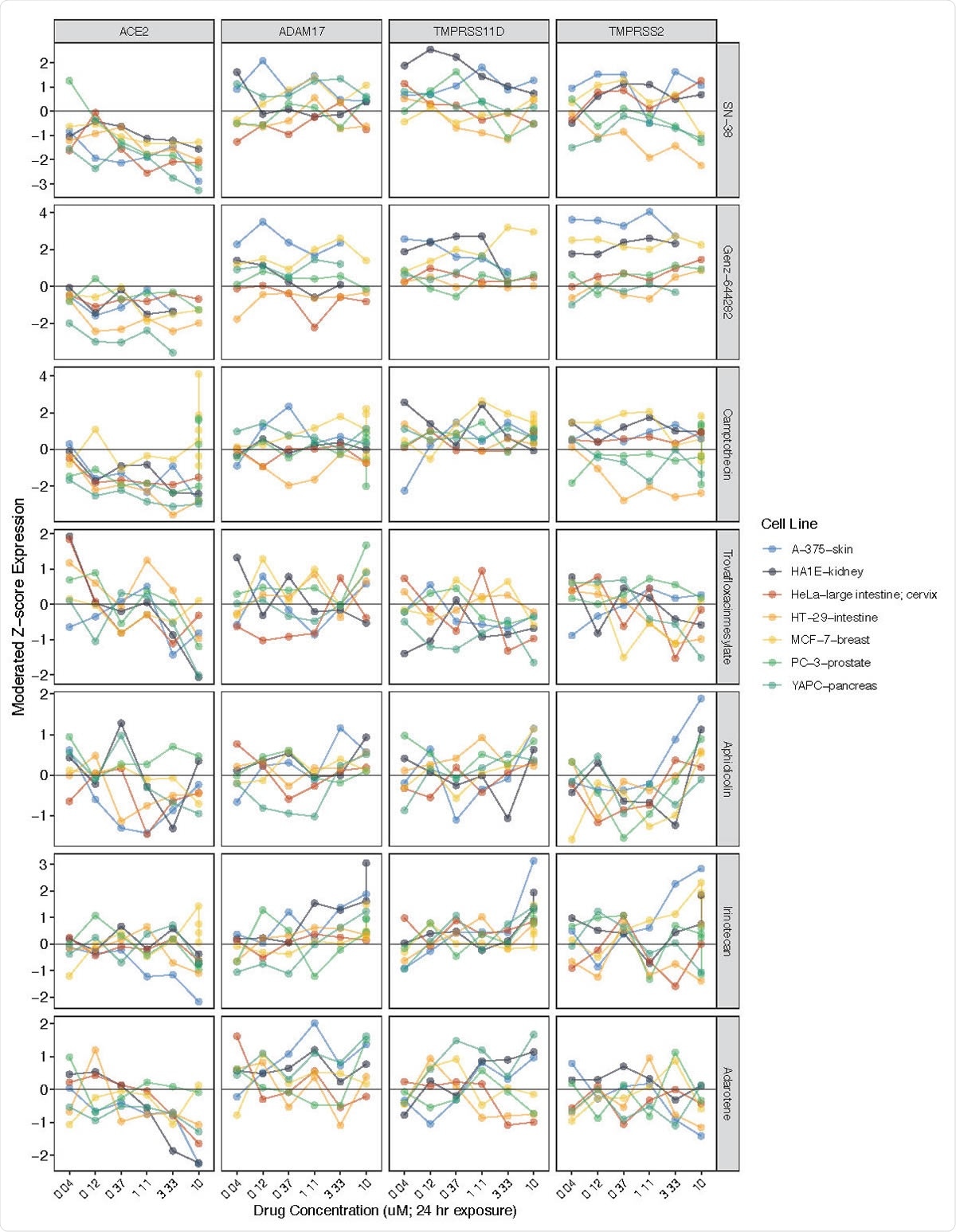
ACE2 expression reductions associated with exposure to various topoisomerase inhibitors. Related genes (ADAM17, TMPRSS11D, TMPRSS2) included for comparison. Y-axis (left) = moderated Z-scores summarizing differential expression across multiple replicates per cell line calculated by the LINCS project. Y-axis (right) = drug name.
Other Factors that Reduce ACE2 Expression
Other covariates that were associated with higher odds of positive COVID-19 testing included belonging to a non-white race, at twice the odds; having blood cancer, at almost three times the odds; and metastatic disease, at over 1.5 times the odds. This showed that several cytotoxic agents are able to reduce ACE2 expression, and thus potentially prevent or treat the infection. The actual effect of taking these drugs on the course of the infection in these patients is unknown since in all cases, they were withheld once the patient tested positive for SARS-CoV-2.
Implications
The findings agree with earlier studies that have proposed the use of these drugs for the treatment of COVID-19, such as a combination of irinotecan and etoposide for critically ill patients with this condition. This was based on the recorded capacity of these drugs to regulate immunity and viral suppression. In addition, it has been shown that some topoisomerases are essential for the virus to replicate.
The study is based on the computer-modeled reduction of ACE2 by these drugs, and not on experimental results. Secondly, clinical validation is required for the concept that ACE2 expression is a therapeutic target. Thirdly, the clinical data is too sparse to allow the impact of this therapy to be estimated in real terms. Despite these limitations, the study indicates that these drugs are worthy of further study as potential COVID-19 preventives.
Competing Interest Statement
Lead author James Robert White is the founder and owner of Resphera Biosciences, LLC. JJ holds a patent licensed by MDSeq, Inc. GA has received honoraria for advisory roles from Hoffman La-Roche, Bayer and Servier and honoraria for speaking engagements from Hoffman La-Roche, Bristol Myers Squibb, Bayer and Servier. GA has received travel grants from Hoffman La-Roche, Bayer, Servier, Amgen and Merck and research funds have been awarded to GA from Bayer. GA is an uncompensated advisor for Menarini and Treos Bio, Inc. LAD is a member of the board of directors of Personal Genome Diagnostics (PGDx) and Jounce Therapeutics. LAD is a paid consultant to PGDx, 4Paws and Neophore. LAD is an uncompensated consultant for Merck but has received research support for clinical trials from Merck. LAD is an inventor of multiple licensed patents related to technology for circulating tumor DNA analyses and mismatch repair deficiency for diagnosis and therapy from Johns Hopkins University. Some of these licenses and relationships are associated with equity or royalty payments directly to Johns Hopkins and LAD. LAD also holds equity in PGDx, Jounce Therapeutics, Thrive Earlier Detection and Neophore, and his spouse holds equity in Amgen. The terms of these arrangements for LAD are being managed by Johns Hopkins and Memorial Sloan Kettering in accordance with their conflict of interest policies.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.