Although it is clear that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of the current COVID-19 pandemic, primarily causes dysfunction of the respiratory system, growing evidence indicates its effects on other organ systems, including our central nervous system (CNS). Research conducted in COVID-19 disease patients has proven that a subset exhibit neurologic symptoms, but it is not clear if SARS-CoV-2 directly affects the CNS or if the neurological symptoms are linked to secondary immune-mediated mechanisms.
Details of the study
COVID-19 patients admitted to Yale New Haven Hospital with neurological symptoms were enrolled in this study. The patients were undergoing clinical lumbar puncture and consented to the additional CSF collection for research purposes.
Age and sex-matched people who were SARS-CoV-2 negative served as controls. CSF and blood samples were processed for use in single-cell RNA sequencing and were assessed for antibodies using ReScan, a custom phage-display library containing 9 SARS-CoV-2 antigens. For studies in a mouse model, 6 to 12-week old mice of both sexes were used.
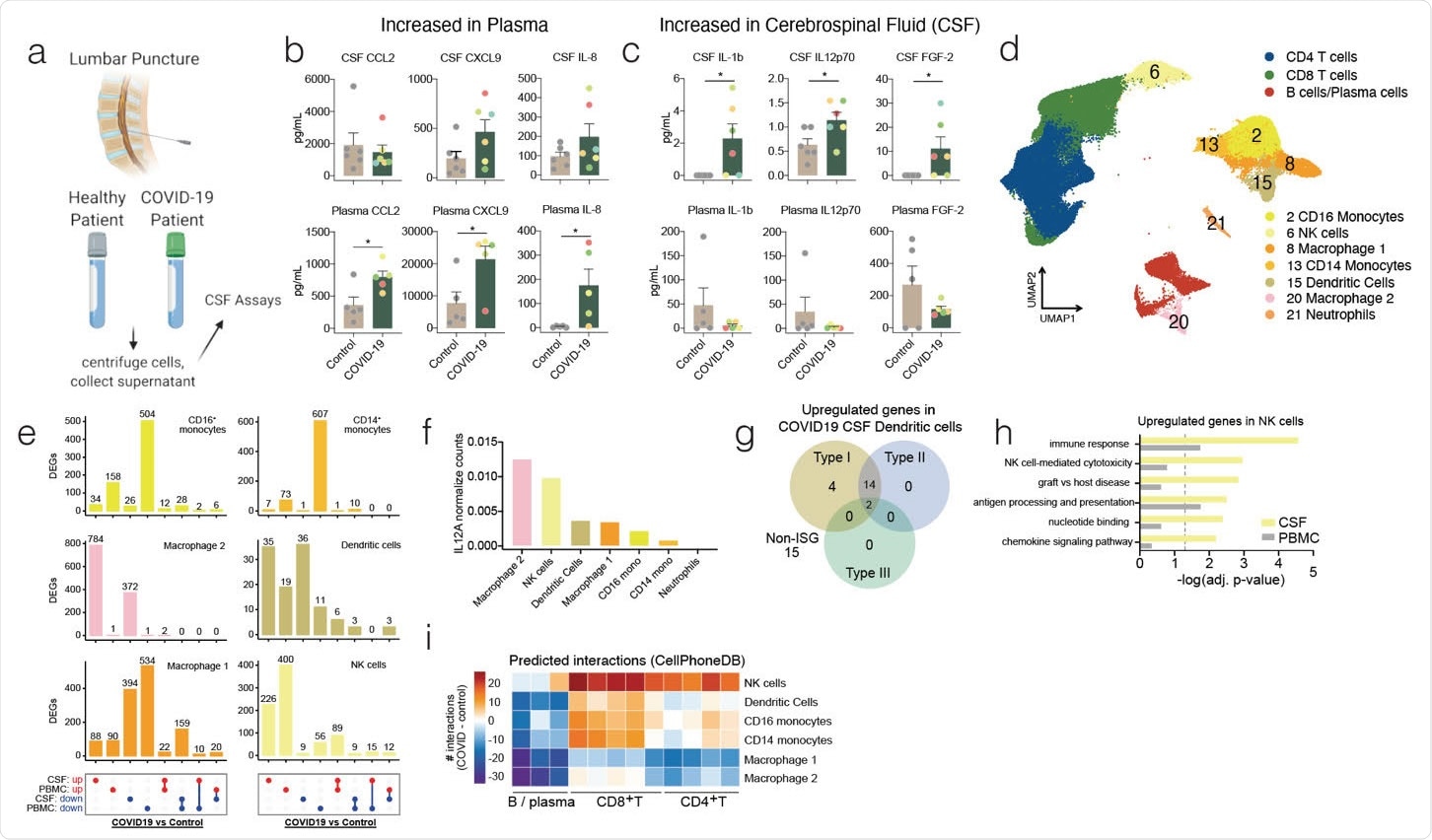
Distinct immunological landscape of COVID-19 patient’s CSF versus PBMC. (a) Schematic of study design. CSF and blood was collected from healthy and COVID-19 patients and cells were collected along with the CSF supernatant and plasma for downstream analysis. (b and c) Luminex based cytokine profiling of CSF (Top row) and plasma (Bottom row) of control and COVID-19 patients. b, cytokines significantly increased in plasma of COVID-19 patients but not in the CSF. c, cytokines significantly increased in CSF of COVID-19 patients but not in the plasma. Color of dots indicate unique patient identity(d) UMAP projection of 10x single cell RNA-sequencing of CSF and PBMC of healthy and COVID-19 patients. (e) UpSet plot showing differentially expressed genes (DEGs) in innate immune cells of COVID-19 patients versus healthy patients in CSF and PBMC. (f) Normalized counts of IL12A transcripts on a population level for innate immune cells. (g) Interferome analysis of upregulated differentially expressed genes in dendritic cells of COVID-19 patient CSF compared to healthy patient CSF. (h) Gene ontology enrichment of genes upregulated in NK cells of COVID-19 patients in both the CSF and PBMC. (i) CellPhoneDB analysis of interaction between innate immune cells and adaptive immune cells by clustering shown in Fig 1d. Number of interactions between the cells in the CSF of COVID-19 patients were subtracted by the number of interactions in the CSF of control patients to derive the heatmap. Cytokine data is derived from n = 6 for control and COVID-19 CSF, and n = 5 for control and COVID-19 plasma. Single cell RNA-seq is derived from a total of 16 libraries generated by us, and 8 additional controls from a previously published data set (n = 3 for control CSF and PBMC, n = 5 for COVID-19 CSF and PBMC, and n = 8 from Gate et al 2020). Two-tailed unpaired t-test was performed (*, P<0.05).

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Findings of the study
All patients studied had anti-SARS-CoV-2 antibodies in the CSF, but there were striking differences in the specificity of the antibody epitope in the CSF and the prevalence of B cell receptor sequences. The team found a significant presence of B cells in the CSF of COVID-19 patients compared to that in controls. Single-cell RNA sequencing showed several B cell subtypes in the CSF and peripheral blood mononuclear cells (PBMCs).
“We tested whether viral invasion into the CNS may lead to a compartmentalized antibody response using a mouse model we recently developed that reliably dissociates pulmonary and neurological infection of SARS-CoV-213,” wrote the authors.
Studies of SARS-CoV-2 infection in mice CNS showed localized and compartmentalized CNS antibody response following viral neuroinvasion. For a SARS-CoV-2 infection localized to the lung, there were increased antibody responses in the lung and serum of the mice and very low antibody presence in the brain or CSF. On the intranasal administration of SARS-CoV-2, there were increased antibodies in all 4 compartments - serum, lung, brain, and CSF. Finally, on intracranial SARS-CoV-2 administration, the antibody response was more robust in the brain and CSF and absent in the serum and lung of the mice.
What the findings mean for COVID-19 patients?
CSF is produced in the brain and is the only CNS tissue surrogate that can be readily sampled in humans. With the help of CSF and blood assessment in acute COVID-19 patients with neurological symptoms, the team was able to show compartmentalized CNS immune response to SARS-CoV-2 infection.
The results of this study highlight the CNS-specific immune responses to SARS-CoV-2 infection and emphasize the importance of informed treatment of neurological symptoms linked to COVID-19. These findings demonstrate that CSF is a better surrogate than serum for studying the response of the brain to SARS-CoV-2 infection. They also show that CNS antibody responses can occur independently of and in the absence of systemic infection.
The team noted unique patterns in the T and B cell repertoires of CSF cells. Although there was some overlap between the top T and B cell clones in circulation and CSF, the most abundant T and B cell clones were more or less unique to each compartment.
“We find an increase in CSF but not plasma IL-12 and IL-1b, cytokines central for coordinating innate and adaptive immune responses to invading pathogens, and further find transcriptional evidence for the role of IL-12 and IL-1 in contributing to the CNS immune response to SARS-CoV-2, with transcriptional changes in CSF effector T cells being enriched for responses to IL-12 and IL-1.”, say the authors.
The CNS antibodies were different from that present in serum. The presence of the SARS-CoV-2 antibody in the CSF of COVID-19 patients clearly shows viral infection of the CNS. Overall, the results of the study show that neurological symptoms in COVID-19 patients may be partly due to a separate immune response within the CNS and that COVID-19 treatment may benefit from targeted therapies that focus on compartmentalized immune responses.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Immunologically distinct responses occur in the CNS of COVID-19 patients Eric Song, Ryan D Chow, Ruoyi Jiang, Colin R. Zamecnik, Rita P Loudermilk, Yile Dai, Feimei Liu, Bertie Geng, Jennifer Chiarella, Benjamin Israelow, Arnau Casanovas-Massana, Albert I Ko, Aaron Ring, Steven H. Kleinstein, Serena Spudich, Michael R Wilson, Akiko Iwasaki, Shelli F. Farhadian bioRxiv 2020.09.11.293464; doi: https://doi.org/10.1101/2020.09.11.293464
- Peer reviewed and published scientific report.
Song, Eric, Christopher M. Bartley, Ryan D. Chow, Thomas T. Ngo, Ruoyi Jiang, Colin R. Zamecnik, Ravi Dandekar, et al. 2021. “Divergent and Self-Reactive Immune Responses in the CNS of COVID-19 Patients with Neurological Symptoms.” Cell Reports Medicine 2 (5): 100288. https://doi.org/10.1016/j.xcrm.2021.100288. https://www.cell.com/cell-reports-medicine/fulltext/S2666-3791(21)00116-6?.