The current COVID-19 pandemic is caused by an enveloped single-stranded RNA virus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It comes from the genus of betacoronaviruses that also includes the two highly pathogenic viruses that caused earlier epidemics of respiratory illness - SARS-CoV and MERS-CoV – as well as two other human coronaviruses (CoV), HCoV-OC43, and HCoV-HKU1.
The current SARS-CoV-2 has an RNA sequence, which is ~80% similar to that of SARS-CoV, and thus many of the antivirals that seem to be active against it were first developed against either the latter or other CoV. One such drug category is the Main Protease inhibitors.
The Importance of Mpro
SARS-CoV-2 enters the host cell by engaging with the angiotensin-converting enzyme 2 (ACE2) receptor, expressed on the target host cells. Following its attachment to the receptor, it enters the cell via membrane fusion or by internalization within endosomes. Within the host cell cytoplasm, the viral RNA is translated into two large polyproteins. These are cleaved into several nonstructural proteins, including the two viral proteases, the main protease (Mpro), also called 3-chymotrypsin-like protease (3CLpro), and the papain-like protease (PLpro).
Along with other essential enzymes like the viral RNA-dependent RNA polymerase (RdRp) complex, these proteases are crucial for viral replication. Any interference with any part of the viral life cycle will, therefore, also stop viral replication. Thus, one of the most important drug targets in the spotlight of research against SARS-CoV-2 is the Mpro.
The Mpro is a cysteine protease that produces 11 or more cuts in the large viral polyprotein. It is also suited to inhibition by compounds that spare the host cell proteases because of the unique preference for a P1 glutamine on the substrate. This preference is shared by many other viral MPro enzymes, including rhinovirus, norovirus, and enterovirus.
Specific Inhibitors of Mpro
As a result, several potent inhibitors of this enzyme have been designed, all of which contain a glutamine-mimetic substitution of 2-pyrrolidone at P1. These include the human rhinovirus Mpro inhibitors Rupintrivir (AG7088) and AG7404, and GC376, a broad-spectrum antiviral inhibiting both SARS-CoV and MERS-CoV in vitro. In vivo studies in cats infected with the feline infectious peritonitis virus have confirmed its antiviral activity.
Novel Inhibitors
Other novel compounds with Mpro inhibitory activity have also been claimed to have antiviral activity. These include ebselen, disulfiram, tideglusib, carmofur, shikonin, and PX-12. A new research paper by scientists at the University of Arizona and the National and Kapodistrian University of Athens and published on the preprint server bioRxiv* discusses the role of these molecules as potential Mpro inhibitors in the prevention of SARS-CoV-2 infection.
Ebselen has been noted to have anti-inflammatory and anti-oxidant activity. Both ebselen and its analogs have anti-Mpro and anti-PLpro activity. However, it is reported to have non-specific activity against many unrelated proteins as well.
Disulfiram also has broad inhibitory activity against several enzymes like methyltransferase, urease, and kinase via their cysteine residues. It has been reported to inhibit SARS-CoV and MERS-CoV PLpro at micromolar concentrations. Still, this effect is easily nullified by β-mercaptoethanol, a reducing agent that is added to a typical enzymatic assay.
Carmofur inhibits the human acid ceramidase via covalent modification, while PX-12 inhibits tubulin polymerization. Tideglusib is an irreversible inhibitor of glycogen synthase kinase-3β (GSK-3β).
Testing for Inhibitory and Antiviral Activity
Among the six compounds, ebselen alone inhibited SARS-CoV-2 replication at micromolar concentration, while disulfiram reduced it by a third. The researchers aimed to find if these compounds can specifically inhibit Mpro, or do they just inactivate it by any of several non-specific mechanisms? If so, does their antiviral activity in the host cell mirror the potency of enzyme inhibition in terms of their inhibitory concentration (IC50)?
The researchers, therefore, tested the six compounds for inhibitory activity against a panel of viral cysteine proteases, namely, the SARS-CoV-2 Mpro and PLpro, the 2A protease (2Apro), and 3C protease (3Cpro) from EV-A71 and EV-D68with and without the addition of the reducing agent DTT. Of these, EV-A71 3Cpro and EV-D68 3Cpro have similar folding properties as Mpro, with the others being dissimilar.
They used multiple assays, including enzymatic assay, thermal shift binding assay, and native mass spectroscopy (MS) assay.
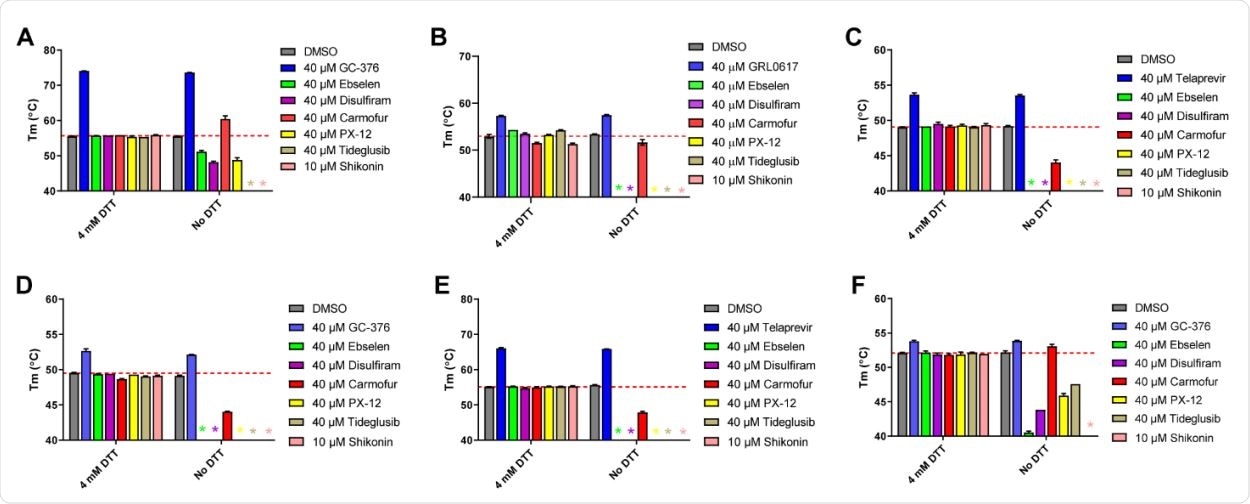
Thermal shift binding assay of SARS-CoV-2, EV-A71, and EV-D68 proteases against inhibitors investigated in this study. (A) SARS-CoV-2 Mpro; (B) SARS-CoV-2 PLPro; (C) EV-A71 2Apro; (D) EV-A71 3Cpro; (E) EV-D68 2Apro; and (F) EV-D68 3Cpro. 3 µM protease in its corresponding enzymatic reaction buffer in the presence of 4 mM DTT or in the absence of DTT was pre-incubated with DMSO or 40 µM protease inhibitors (shikonin was tested at 10 µM because 40 µM Shikonin completely quenches SYPRO orange dye fluorescence signal) at 30 °C for 30 min. The melting temperature (Tm) was calculated as the mid log of the transition phase from the native to the denatured protein using a Boltzmann model.32 * indicates that a fluorescence peak was not observed in the melting curve; red dash line shows the protease Tm with DMSO in the presence of 4 mM DTT.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Non-specific Inhibition
They found that in the presence of DTT, none of them were able to inhibit Mpro. However, without DTT, all six had broad inhibitory activity against all the viral proteases, especially against the 2Apro and 3Cpro from EV-A71 and EV-D68. This indicates the occurrence of non-specific inhibition due to alkylation or oxidation of the cysteine residue.
The addition of a reducing agent prevents such effects, which are expected to be unaltered in the case of a specific cysteine protease inhibitor.
Secondly, they observed through molecular dynamics (MD) simulations that these compounds bind to SARS-CoV-2 Mpro at low affinity, supporting their non-specific mechanism of inhibition. Only carmofur possibly has specific activity against Mpro, but low potency.
Thus, they sum up: “We provide compelling evidence suggesting that ebselen, disulfiram, tideglusib, carmofur, shikonin, and PX-12 are non-specific SARSCoV-2 Mpro inhibitors.”
No Cellular Antiviral Activity
Again, these compounds failed to suppress the replication of either of these viruses at the highest concentration that was consistent with safety. In other words, they concluded, “The enzymatic inhibition potency of cysteine protease inhibitors obtained in the absence of DTT cannot be used to predict the cellular antiviral activity.”
Despite the urgent need for effective antivirals to contain the ongoing pandemic, scientists still need to observe rigor to identify specific antiviral compounds rather than broader hits. Thus, any promising compounds should be tested early in the course of development by secondary assays to rule out the non-specific activity.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Ma, C. et al. (2020). Ebselen, Disulfiram, Carmofur, PX-12, Tideglusib, And Shikonin Are Non-Specific Promiscuous SARS-Cov-2 Main Protease Inhibitors. bioRxiv preprint. doi: https://doi.org/10.1101/2020.09.15.299164. https://www.biorxiv.org/content/10.1101/2020.09.15.299164v1
- Peer reviewed and published scientific report.
Ma, Chunlong, Yanmei Hu, Julia Alma Townsend, Panagiotis I. Lagarias, Michael Thomas Marty, Antonios Kolocouris, and Jun Wang. 2020. “Ebselen, Disulfiram, Carmofur, PX-12, Tideglusib, and Shikonin Are Nonspecific Promiscuous SARS-CoV-2 Main Protease Inhibitors.” ACS Pharmacology & Translational Science 3 (6): 1265–77. https://doi.org/10.1021/acsptsci.0c00130. https://pubs.acs.org/doi/10.1021/acsptsci.0c00130.