Researchers are exploring various avenues to develop effective antivirals and vaccines to contain the current COVID-19 pandemic. Drugs that have been approved for other indications are being repurposed for use against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Among them is the combination of lopinavir and ritonavir, which have been in use in an attempt to reduce the severity of the disease. However, a new South Korean study published on the preprint server bioRxiv* in September 2020 shows that this combination is ineffective against the virus.
Lopinavir is a protease inhibitor and was approved for the treatment of HIV-1 infection. Ritonavir inhibits the cell enzyme cytochrome P450, which metabolizes lopinavir. This markedly boosts the plasma concentration of lopinavir. Lopinavir was investigated as a potential treatment for SARS-CoV, the coronavirus which caused an outbreak of severe acute respiratory illness in 2002.
Early in vitro studies indicated that lopinavir showed promise, inhibiting the SARS-CoV. With an IC50 of 25~50 μM, this showed inhibitory activity against the SARS-CoV Mpro. Clinical studies showed that when combined with ritonavir, the risk of infection with this virus was lower. When the Middle East Respiratory Syndrome (MERS) outbreak occurred, the same combination was found to have therapeutic efficacy against this infection as well.
SARS-CoV-2 has close phylogenetic relationships and highly similar genomic sequences with these viruses, particularly SARS-CoV. This prompted early trials of this drug combination in the treatment of COVID-19 as well. However, despite early and widespread use, it has not commended itself by universally impressive results.
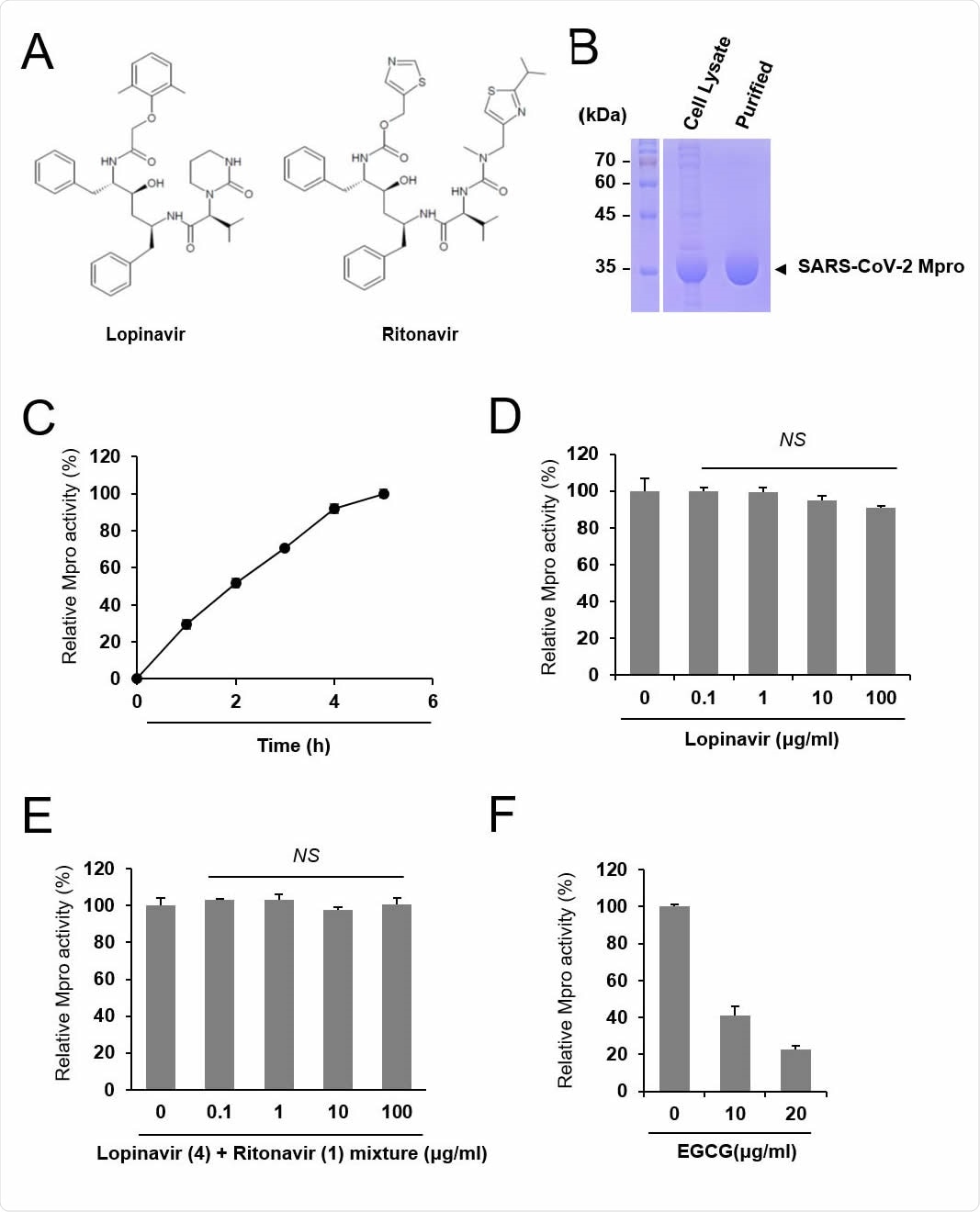
Lopinavir is ineffective to inhibit SARS-CoV-2 Mpro. (A) Chemical structure of lopinavir and ritonavir. (B) Purification of SARS-CoV-2 Mpro. Cell lysate and purified Mpro protein were subject to SDS-PAGE. (C) Measurement of the relative Mpro activity over time. Activity is measured relative to maximal activity at 5 hr. (D) Lopinavir did not inhibit Mpro. Indicated concentrations of lopinavir were incubated with Mpro and the relative protease activity was determined. The Mpro activity was measured in quadruple and the mean and standard deviation are shown. NS, not significant. (E) Mixtures of lopinavir and ritonavir at a weight ratio of 4:1 were incubated with Mpro, and the relative protease activity was determined. (F) EGCG was incubated with Mpro, and the protease activity was examined as a positive control.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Reasons for Lack of Efficacy
As stated earlier, lopinavir is a protease inhibitor and is thought to inhibit the main protease or Mpro of the SARS-CoV. Using bioinformatics data, researchers consider that it may also engage with SARS-CoV-2 Mpro. This would lead to inhibition – or so it was assumed. However, this was likely an unfounded assumption.
The researchers in the current study examined the inhibitory activity of both the drugs on purified Mpro protein in vitro, using a protease assay. They used purified Mpro from bacteria, which was then mixed with lopinavir at various concentrations. They then assessed the protease activity of the enzyme.
The researchers observed that even at concentrations of up to 100 μg/ml, there was no significant reduction in the catalytic activity of Mpro. They then looked at a combination of both drugs, similar to those used in clinical trials. They found the same result.
However, when they mixed the purified Mpro with the control inhibitory compound EGCG, the result was significant inhibition of Mpro activity.
The findings indicate that lopinavir and ritonavir do not inhibit the activity of Mpro, which explains why clinical trials on these drugs in COVID-19 have consistently failed to produce any evidence of efficacy, unlike those performed in SARS patients. The researchers point out, “This study highlights differences between the highly related SARS, MERS, and COVID-19-causing coronaviruses, and will be helpful in the selection of drug candidates for current and future COVID-19 clinical tests.”
Amino Acid Structures May Hold the Key
The researchers then looked at structural differences between SARS-CoV Mpro and SARS-CoV-2 Mpro. Using earlier computational analysis data, they were able to see the amino acid residues that were different in the two enzymes. They also identified the amino acids that might be taking part in the Mpro-lopinavir interaction.
Intriguingly, over 96% of these amino acids are identical to those in SARS-CoV-2 Mpro, with just one at the lopinavir-Mpro interaction site being different. At position 46 of the putative sequence, serine replaces alanine in SARS-CoV-2 Mpro, relative to SARS-CoV and MERS-CoV Mpro. This may seem to be an insignificant difference but could be the reason why the viral Mpro fails to respond to lopinavir inhibition. The researchers say that further research on the structure of this enzyme will be needed to uncover the implications of such changes in the amino acid sequence.
Implications
The importance of the in vitro study carried out here is the immense saving of time, human effort, and costs on clinical trials, which would have turned out to be futile given the lack of inhibitory activity of these drugs on SARS-CoV-2 Mpro. Such findings will be of great help in designing successful trials.
The study concludes, “We demonstrated that lopinavir-ritonavir is not effective in inhibiting SARS-CoV-2 Mpro, and this information will be useful to choose the right drug for SARS-CoV-2 clinical trials.”
Source

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.