A recent study by French researchers has demonstrated that experimental exposure to severe acute respiratory coronavirus 2 (SARS-CoV-2) is capable of inducing mild infection in hamsters and ferrets within 7 – 10 days of exposure. However, the infection completely resolves within 14 days. The study is currently available on the bioRxiv* preprint server.
SARS-CoV-2, the causative pathogen of coronavirus disease 2019 (COVID-19) pandemic, emerged in December 2019 in China, most probably due to zoonotic transmission from an animal to human. Later, the virus has gained the ability to transmit from human to human.
Understanding the pathogenesis and nature of SARS-CoV-2 infection is the key to identify effective therapeutic and preventive interventions to attenuate the COVID-19 trajectory. In this context, non-human primates, such as monkeys, cats, hamsters, and ferrets, serve as suitable experimental models to study the pathogenesis of SARS-CoV-2 infection.
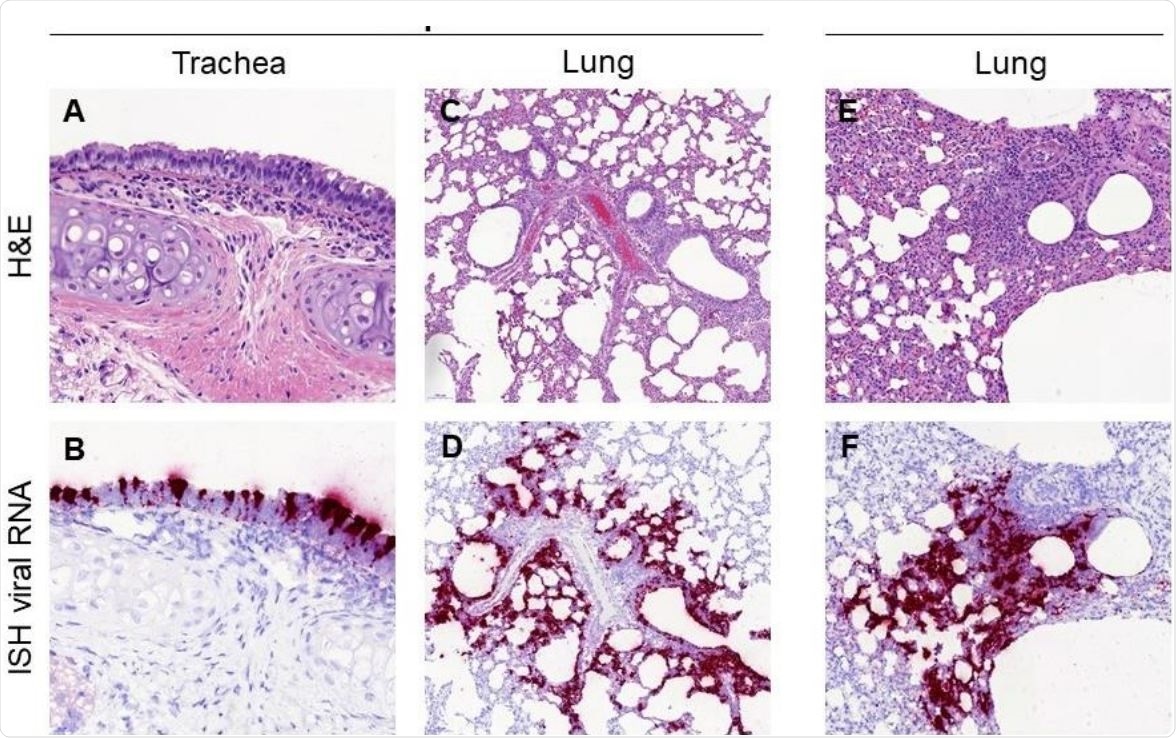
Histopathological findings in hamsters inoculated with SARS-CoV-2 UCN19 strain. Mild inflammatory cell infiltration observed in the trachea at D2 (A, 400x) with high presence of viral RNA by ISH within respiratory epithelial cells (B, 400x). Inflammatory infiltrates with the lung parenchyma, mostly within the bronchial and bronchiolar mucosa but also surrounding airways and blood vessels are observed at D2 (C, 100x) and D4 (E, 200x). The presence of the inflammatory infiltrates is correlated with the viral RNA staining in sequential sections at D2 (D, 100x) and D4 (F, 200x).

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Current study design
In this study, the scientists aimed at evaluating the pathogenicity, immune responses, and clinical outcomes in hamsters and ferrets infected with low doses of a low-passage SARS-CoV-2 clinical isolate.
According to the scientists, the study will help identify appropriate animal models for developing therapeutic interventions against SARS-CoV-2 infection.
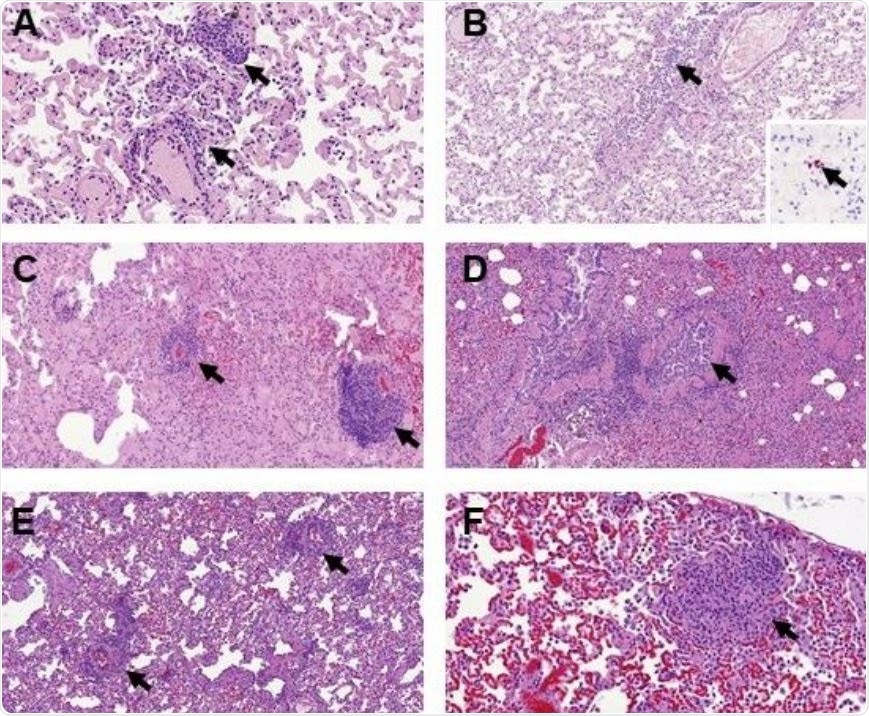
Histopathological findings in the lungs of ferrets inoculated with the UNC19 SARS-CoV-2 strain. Perivascular cuffing (A, 400x, arrows) and mild bronchiolitis (B, 200x, arrow) was observed at D2, with minimal presence of viral RNA (B, insert, 400x) within alveolar walls not related to histopathological lesions. Perivascular cuffing was also observed at D4 (C, 200x, arrows) and D7 (E, 200x, arrows). Mild bronchiolitis with presence of intraluminal inflammatory infiltrates was observed at D4 (D, 200x, arrow). Scattered foci of parenchymal inflammation were also observed at D7 (F, 400x, arrow)
Important observations
Regarding clinical symptoms, some ferrets developed lathery after 7 days of infection, and none of the infected animals had hyperthermia. No change in body weight was observed in ferrets; however, an average increase in body weight was observed in all hamsters. Most importantly, none of the infected animals died due to SARS-CoV-2 infection.
The viral RNA was detected in many tissues (respiratory tissues, liver, spleen, kidney, intestine, olfactory bulb, and spinal bulb) within 2 days of infection, mostly resolved after 14 days of infection.
The viral load was maximum in the nasal turbinate, trachea, and lungs. However, the tissue variability of the viral load was higher in ferrets than hamsters.
The infectious virus was present in the nasal turbinate, trachea, and lungs of all experimental hamsters on day 2 and day 4 of infection. However, in ferrets, no infectious virus was detected in the lungs.
In infected hamsters, the infiltration of macrophages, lymphocytes, and neutrophils was observed in the lungs within 2 – 7 days of infection. In the trachea, mild inflammatory infiltration and mild epithelial cell necrosis were observed between day 2 and day 7 of infection.
In ferrets, inflammatory infiltration of neutrophils, macrophages, lymphocytes, and eosinophils was observed along with mild bronchiolitis within the bronchiolar laminae at day 2 of infection.
Regarding serological characteristics, the presence of immunoglobulin G (IgG) was observed after 7 and 10 days of infection in hamsters and ferrets, respectively.
In hamsters, the level of IgG remained high until day 14 of infection; however, in ferrets, a more gradual increase in IgG level was observed from day 10 of infection.
Neutralizing antibodies were detected in hamsters and ferrets after 7 and 10 days of infection, respectively. However, there was no correlation between the IgG levels and neutralizing antibody titers.
In hamsters, the level of IgG remained stable until day 14 of infection, whereas the neutralizing antibody titers reduced by 50% between day 7 and day 14. However, in one ferret, the neutralizing titers reduced by 50% at day 14 of infection, whereas in another ferret, the titers doubled from day 10 to day 14.
Current study significance
Unlike other animal studies, the current study used relatively lower doses of SARS-CoV-2, which is more comparable to natural infection in humans.
The study shows that a low dose of SARS-CoV-2 exposure through intra-nasal route is capable of inducing mild COVID-19 symptoms in hamsters. Compared to hamsters, ferrets require higher doses of viral inoculation to induce similar levels of lung infection.
The serological observations suggest that both hamsters and ferrets successfully develop robust adaptive immune responses against SARS-CoV-2 soon after viral exposure, which is accompanied by the disappearance of infectious virus.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources