Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection causes coronavirus disease 2019 (COVID-19), which manifests with pulmonary symptoms that show up as diffuse alveolar damage (DAD), pneumocyte hyperplasia and proliferation, excess inflammation, and platelet aggregate or thromboemboli formation in histological studies. The mechanisms behind these histological findings are still unclear.
Previous studies published on PubMed showed that single-cell RNA sequencing had revealed extensive expression of viral entry receptor angiotensin-converting enzyme 2 (ACE2) in many cell types. However, single-cell RNA sequencing and immunohistochemistry showed only low levels of SARS-CoV-2 infection in macrophages, type II pneumocytes, neutrophils, and goblet, ciliated, and endothelial cells.
Although studies have reported that analysis of COVID-19 blood samples showed high levels of monocytes, neutrophils, and inflammatory cytokines including interleukin-6 (IL-6), and depletion of lymphocytes, there was no clear information on the cell types that were infected by SARS-CoV-2 or the extent of infection or immune cell status in the lungs of fatal COVID-19 patients.
In a recent preprint paper published on the medRxiv* server, researchers from the University of Pittsburgh School of Medicine, Pittsburgh, and Icahn School of Medicine at Mount Sinai, New York, demonstrated their investigation of fatal COVID-19 patients to assess infection in various cell types and the extent of damage it caused to the lung tissues.
The team performed multicolor staining of lineage cell markers and viral proteins to identify SARS-CoV-2 tropism and outline lung pathobiology in postmortem tissues of 5 patients with fatal SARS-CoV-2 infection.
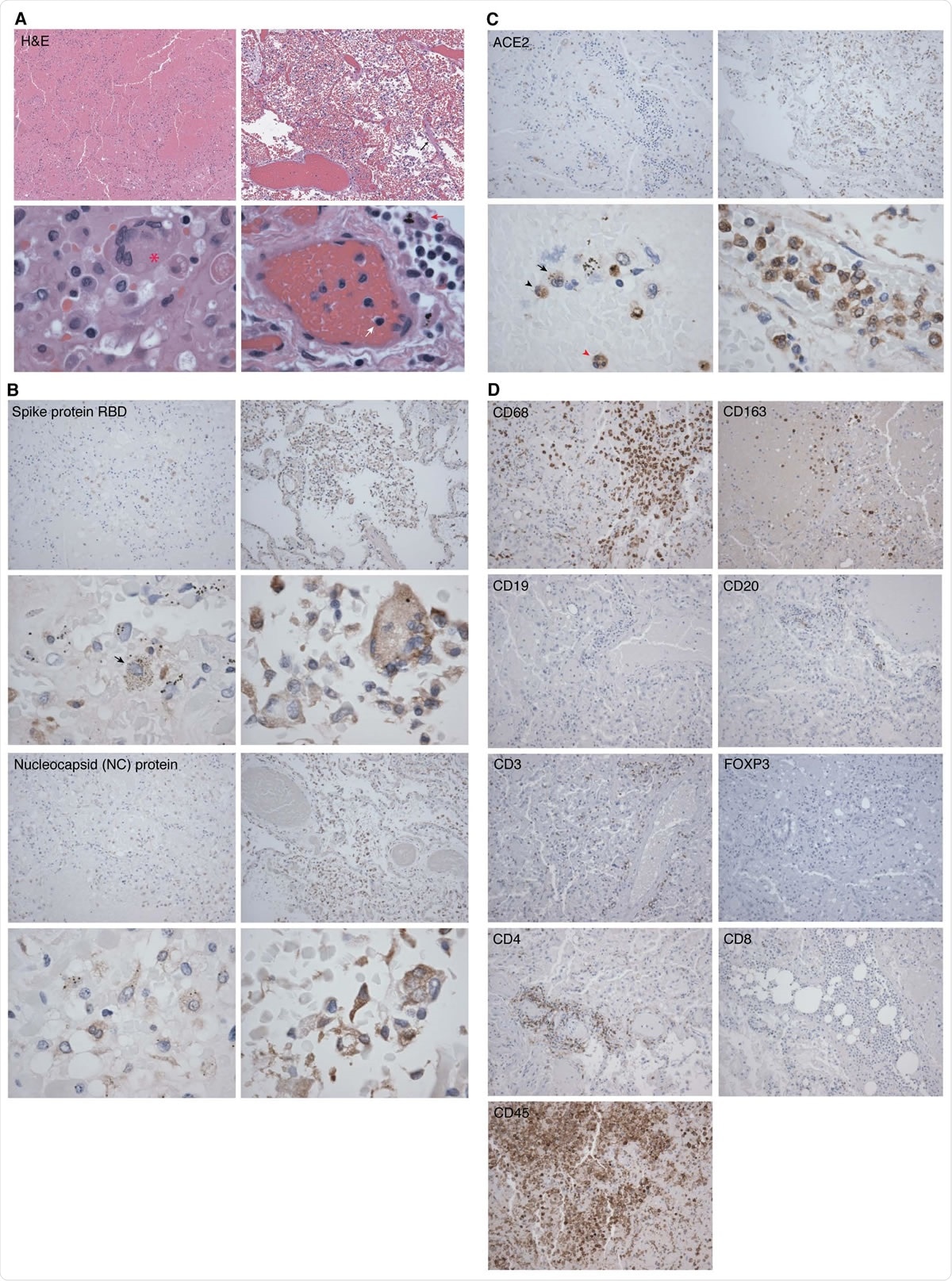
Representative hematoxylin-eosin (H&E) and immunohistochemistry (IHC) staining images of SARS-CoV-2 proteins and markers of immune cells in lung tissues from two COVID-19 patients. Shown are H&E images illustrating significant areas of lung tissues (Panel A, case 2 in left images and case 4 in right images). An image of case 2 showing lung parenchyma with hemorrhagic infarct in top image (100x). A Langhans giant cell is visible in bottom image (asterisk, 600x). An image of case 4 with an early exudative phase of DAD, vascular congestion and rare hyaline membranes in top image (black arrow, 100x), and infiltrations of lymphocytes (white arrow) and macrophages (red arrow) in bottom image (600x). Shown are IHC detection of SARS-CoV-2 infection using antibody against spike protein (receptor binding domain, RBD) and NC protein (100x) (Panel B, case 2 in left images and case 4 in right images). A macrophage infected by SARS-CoV-2 is visible in case 2 bottom image (black arrow, 600x). Case 2 has less positive cells compared to case 4 for both viral proteins. Shown are IHC detection of ACE2 protein expression in lung tissues (Panel C, case 2 in left images and case 4 in right images). Immune cells identified in case 2 in bottom image are a monocyte (black arrowhead), a macrophage (black arrow) and a neutrophil (red arrowhead), all expressing ACE2 protein (600x). Shown are IHC detection of markers of immune cells in a lung tissue from a COVID-19 patient (case 3) consisting of monocytes and macrophages (CD68+ and CD163+), B cells (CD19+ and CD20+), different markers of T cells including T cell receptor (CD3ε+), T regulatory cell (FOXP3), helper T cell (CD4+), cytotoxic T cell (CD8+), and lymphocyte common antigen (CD45+) (100x, Panel D).

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
SARS-CoV-2 infects a wide range of cell types and causes massive tissue damage
The lung parenchyma showed severe diffuse alveolar damage with thromboemboli in all cases. SARS-CoV-2 infection was found in a large number of cells, including alveolar epithelial type II/ pneumocyte type II cells (HT2-280), goblet cells (MUC5AC), ciliated cells (tyr-α-tubulin), endothelial cells (CD31 and CD34), and club-like cells (MUC5B).
Over 90% of infiltrating immune cells, including neutrophils (ELA-2), macrophages and monocytes (CD68 and CD163), natural killer (NK) cells (CD56), T-cells (CD3ε), and B-cells (CD19 and CD20) tested positive for viral proteins. Most infected cells tested positive for angiotensin-converting enzyme 2 (ACE2), the viral entry receptor.
“Most infected cells expressed ACE2 protein, but we also observed some ACE2-negative infected cells, which could be due to low expression level of ACE2 protein outside the detection range of the assay, downregulation of ACE2 protein expression at some stage(s) of SARS-CoV-2 infection, virus cell-to-cell spread, or presence of an alternative SARS-CoV-2 receptor.”
The number of infected and ACE2-positive cells directly correlated with the extent of damage to the tissue. The infected tissues showed a lower number of B cells and plenty of CD3ε+ T cells comprising mainly T helper cells (CD4) and few cytotoxic T cells (CTL, CD8), but no T regulatory cell (FOXP3).
In all cases, antigen-presenting molecule HLA-DR of B and T cells was abundant. Most infected and uninfected cells showed pronounced IL-6 expression that increased with more tissue damage.
Findings of the study can aid development of new therapies
Progressive respiratory dysfunction is a crucial feature of fatal COVID-19. This study maps lung immunopathology of fatal SARS-CoV-2 infections. It reveals broad cell tropism and infection of endothelial, parenchymal, and immune cells, all of which are associated with thromboemboli and massive tissue damage. The team also found evidence for clearance of immunosuppressive T -cells and suppression of cytotoxic T cells and B cells. Other than these, they noticed extensive infiltration and activation of immune cells and pronounced expression of IL-6 in both infected and uninfected cells.
The findings of this study were in agreement with recent studies that showed evidence for the activation of immune cells, cell tropisms, and clearance of immunosuppressive cells in lung tissues of patients who died of COVID-19. All of these pathological changes have the potential to cause severe tissue damage, excess inflammation, thromboemboli, and compromised adaptive immune responses. The team believes that these findings can have vital implications for the development of new therapeutic approaches.
Although studies had earlier reported the detection of SARS-CoV-2 proteins in macrophages by IHC, the authors claim that theirs is the first study to illustrate and quantify SARS-CoV-2 infection in various types of T cells and in neutrophils.
“The results of our study present a direct visualization of the multiple cell types infected by SARS-CoV-2 from patients who died of COVID-19, and offer insight into the pathogenesis of the overwhelming damage found in lung tissues in fatal COVID-19 cases.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Broad SARS-CoV-2 cell tropism and immunopathology in lung tissues from fatal COVID-19 Suzane Ramos da Silva, Enguo Ju, Wen Meng, Alberto E. Paniz Mondolfi, Sanja Dacic, Anthony Green, Clare Bryce, Zachary Grimes, Mary E Fowkes, Emilia M. Sordillo, Carlos Cordon-Cardo, Haitao Guo, Shou-Jiang Gao medRxiv 2020.09.25.20195818; doi: https://doi.org/10.1101/2020.09.25.20195818
- Peer reviewed and published scientific report.
Ramos da Silva, Suzane, Enguo Ju, Wen Meng, Alberto E Paniz Mondolfi, Sanja Dacic, Anthony Green, Clare Bryce, et al. 2021. “Broad Severe Acute Respiratory Syndrome Coronavirus 2 Cell Tropism and Immunopathology in Lung Tissues from Fatal Coronavirus Disease 2019.” The Journal of Infectious Diseases, April. https://doi.org/10.1093/infdis/jiab195. https://academic.oup.com/jid/article/223/11/1842/6219606.