Researchers in the United States have conducted a study showing that anti-sense agents called peptide-conjugated morpholino oligomers (PPMOs) have the ability to specifically and potently suppress the growth of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – the agent responsible for the current coronavirus disease 2019 (COVID-19) pandemic.
David Stein (Oregon State University) and colleagues say the findings demonstrate that the PPMOs readily enter cells and inhibit the replication of the virus in a non-toxic manner.
"The data indicate that PPMOs can potently and specifically suppress SARS-CoV-2 growth and are promising candidates for further pre-clinical development," write the researchers.
A pre-print version of the paper is available on the server bioRxiv*, while the article undergoes peer review.
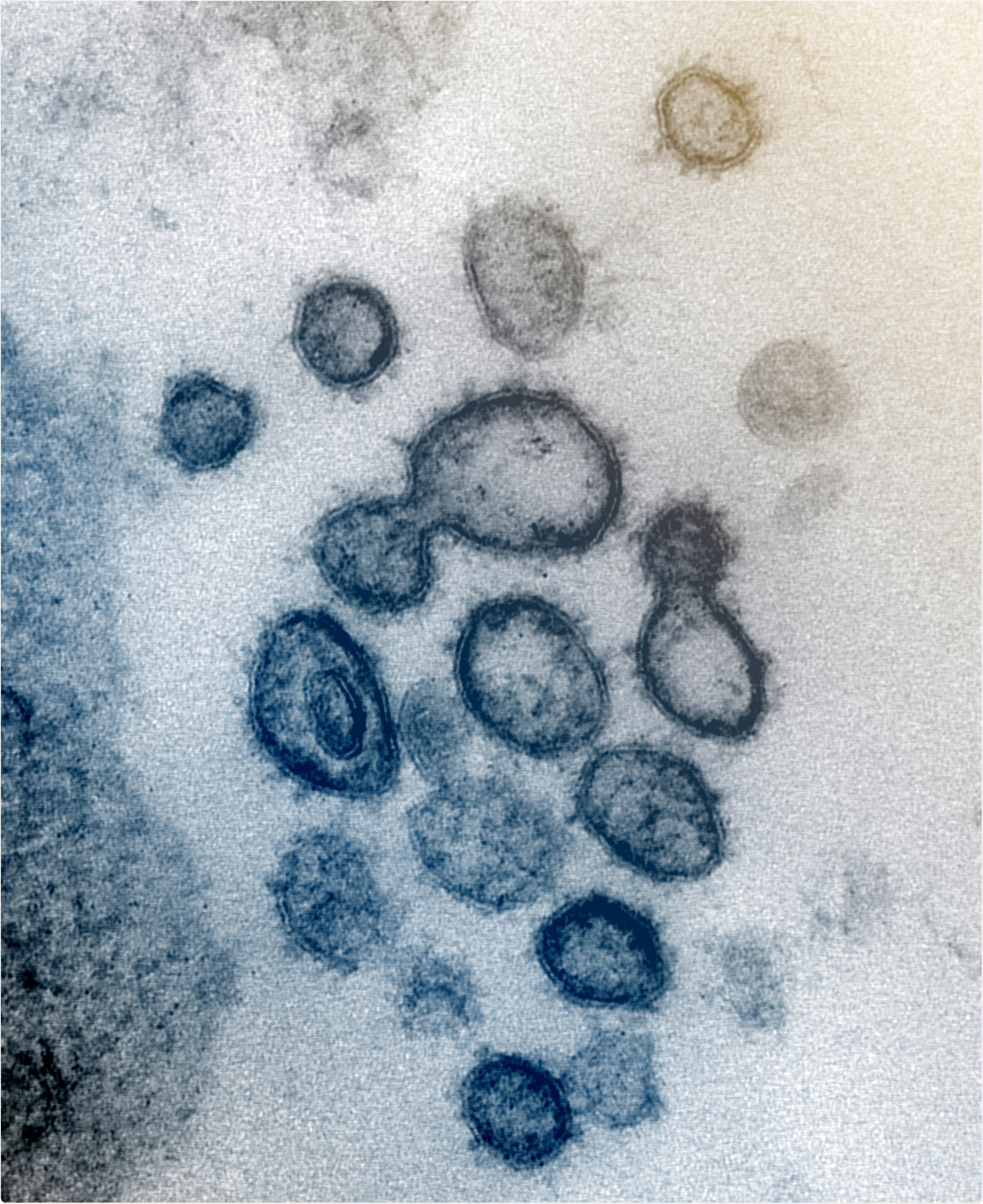
This transmission electron microscope image shows SARS-CoV-2—also known as 2019-nCoV, the virus that causes COVID-19—isolated from a patient in the U.S. Virus particles are shown emerging from the surface of cells cultured in the lab. The spikes on the outer edge of the virus particles give coronaviruses their name, crown-like.Image captured and colorized at NIAID's Rocky Mountain Laboratories (RML) in Hamilton, Montana. Credit: NIAID

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Antiviral therapeutics are urgently needed
Since the first cases of SARS-CoV-2 were first identified in Wuhan, China, late last year, the virus has swept the globe at an unprecedented rate and now infected more than 34.3 million people and caused more than one million deaths.
In the absence of a vaccine and any effective treatments, there is an urgent need to develop innovative direct-acting antiviral agents.
PPMOs are single-stranded-nucleic acid analogs capable of entering cells (without requiring delivery assistance) that interfere with the expression of targeted RNA through sequence-specific steric blocking.
PPMOs have already proved effective as antiviral agents
PPMOs have been shown to be effective across a range of in vitro and in vivo applications.
Several in vivo studies of respiratory viruses, including influenza A virus and respiratory syncytial virus, have shown that intranasally administered PPMOs designed to target viral genome sequences effectively reduced viral titers and disease in lung tissue.
The five prime untranslated region (5' UTR) of the genome in coronaviruses contain sequences that are required for various stages of the virus life-cycle, including translation and RNA synthesis.
In previous studies, PPMOs designed to target various sites in the 5'UTR of SARS-CoV-1 were shown to have antiviral effects.
What did the current study involve?
The researchers synthesized five PPMOs designed to target sequences of genomic RNA in the 5' UTR of SARS-CoV-2. A negative control PPMO random sequence was also synthesized to control any non-specific effects of the PPMO chemistry.
Two of the PPMOs were designed to target the 5'-terminal region of the genome, to interrupt the translation of genomic and subgenomic mRNAs. Two PPMOs were designed to target the genomic 5'UTR region containing the putative SARS-CoV-2 transcription-regulating sequence (TRS) leader region, thereby potentially interfering with base-pairing or translocation. The fifth PPMO was designed to target the polyprotein 1a/b AUG translation start site region, with the intention of blocking translation initiation.
Each PPMO was evaluated for its ability to suppress the viral growth of SARS-CoV-2 in Vero-E6 cell cultures and for its effect on the viability of uninfected cells.
Viral growth was measured by quantitative reverse transcriptase-polymerase chain reaction (RT-PCR), and 50% Tissue Culture Infective Dose (TCID50) assays and cell viability was assessed using adenosine triphosphate (ATP)- based method.
Four of the PPMOs were highly effective at suppressing viral growth
The researchers found that the four PPMOs targeting the 5'terminal region or the TRS-leader region were potent inhibitors of SARS-CoV-2 replication, significantly reducing viral titers at 48 to 72 hours post-infection in a non-toxic and dose-responsive manner.
The PPMO targeting the polyprotein 1a/b AUG translation start site region, on the other hand, was not nearly as effective as the other four PPMOs, writes the team.
"This study demonstrates that PPMO targeted against SARS-CoV-2 can enter cells readily and inhibit viral replication in a sequence-specific, dose-responsive and non-toxic manner," said Stein and colleagues.
"Considering the considerable in vivo antiviral efficacy demonstrated by PPMO against several respiratory viruses in previous studies, further development of the 5'END- and TRS-PPMO appears warranted," concludes the team.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources